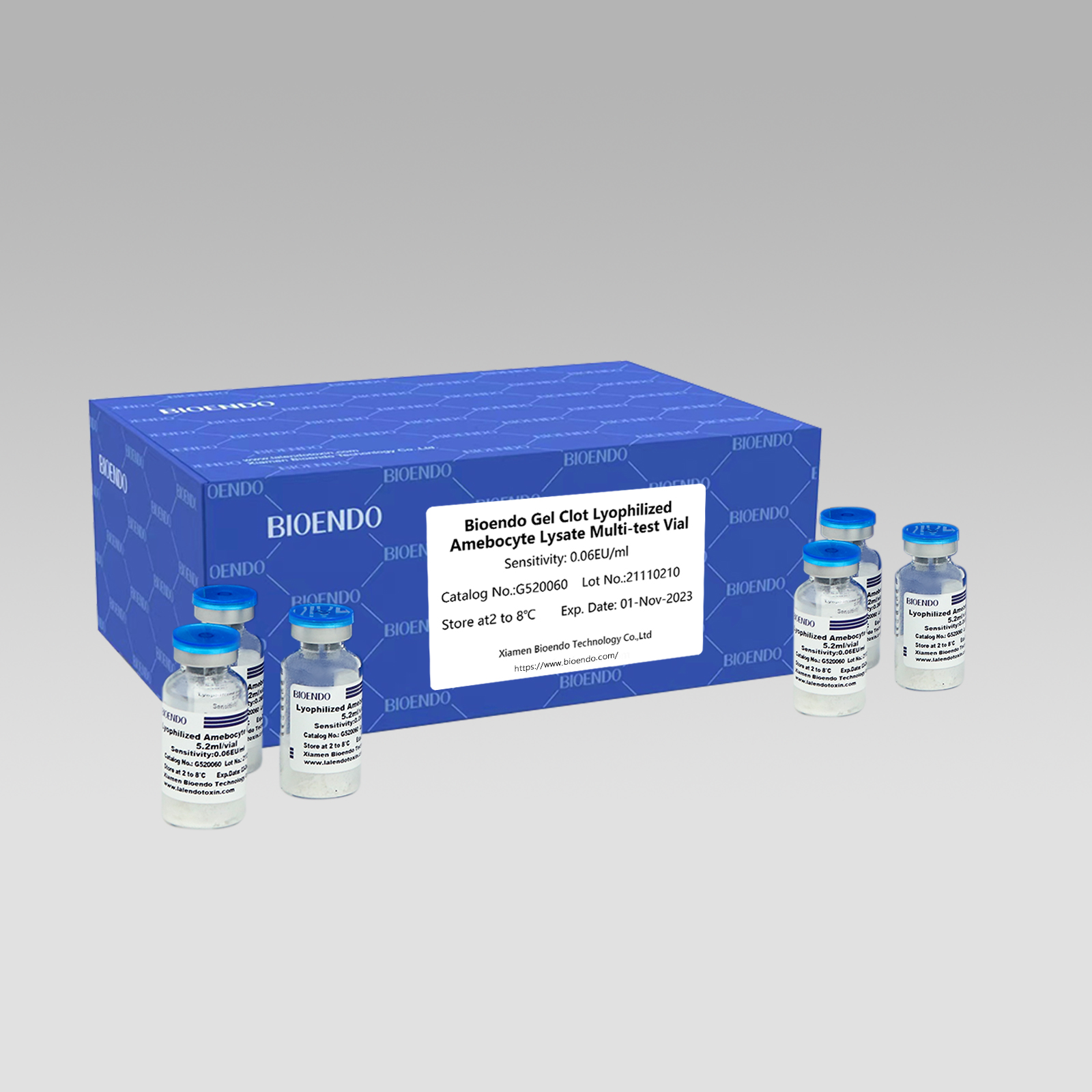
Gel Clot Lyophilized Amebocyte Lysate Multi-test Vial G52
Bioendo G52 series is mainly use in the experiment operation of bacterial endotoxin test as a Bioassay procedure.
1. Product Information
Gel Clot method Lyophilized Amebocyte Lysate Multi-test Vial is the Lyophilized Amebocyte Lysate reagent which select and use gel clot technique to detect endotoxin or pyrogen.
As the widespread method, gel-clot test for endotoxin is simple and does not require specific and expensive instrument. Bioendo provides Gel Clot Lyophilized Amebocyte Lysate - LAL reagent in 5.2ml per vial.
2. Product Parameters
Sensitivity range: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25EU/ml, 0.5 EU/ml
3. Product Application
End-product endotoxin (pyrogen) qualification, water for injection endotoxin assay, raw material endotoxin testing or endotoxin level monitoring during manufacturing process for pharmaceutical companies or medical devices manufacturers.
Note:
Lyophilized Amebocyte Lysate (LAL reagent) manufactured by Bioendo is made from lysate of amebocytes (white blood cells) from the horseshoe crab.
This unique reagent has become an essential tool in the pharmaceutical and medical device industries for the detection of bacterial endotoxins. The horseshoe crab’s amebocytes contain a substance called Lyophilized Amebocyte Lysate, which reacts to bacterial endotoxins by forming a gel-like clot. This reaction is the basis for the LAL test, used to ensure the safety of medical devices, drugs, and other products that come into contact with the human body.
The use of LAL reagent has revolutionized the process of endotoxin detection in the medical field than the Rabit test assay. Its unparalleled sensitivity and specificity make it a crucial component in the quality control and safety assurance of pharmaceuticals, biologics, and medical devices. The LAL test is a rapid and reliable method for endotoxin detection, providing results in as little as 60 minutes. This efficiency allows for quick and accurate decisions regarding the release of products, ultimately enhancing the overall safety and efficacy of medical treatments and devices.
Bioendo’s Lyophilized Amebocyte Lysate (LAL reagent) is produced under strict quality standards to ensure its effectiveness and reliability. The company is dedicated to utilizing sustainable practices in the harvesting of horseshoe crabs to minimize any negative impact on their population. By prioritizing the welfare of these creatures, Bioendo ensures a continued supply of this valuable resource for the production of LAL reagents. Additionally, ongoing research and development efforts are focused on improving the performance and versatility of LAL test endotoxin, further advancing their utility in the medical and pharmaceutical industries.
Gel clot method LAL assay, reconstituted lysate reagent get at least 50 tests per vial:
|
Catalog Number |
Sensitivity (EU/ml or IU/ml) |
ml/vial |
Tests/Vial |
Vials/Pack |
|
G520030 |
0.03 |
5.2 |
50 |
10 |
|
G520060 |
0.06 |
5.2 |
50 |
10 |
|
G520125 |
0.125 |
5.2 |
50 |
10 |
|
G520250 |
0.25 |
5.2 |
50 |
10 |
|
G520500 |
0.5 |
5.2 |
50 |
10 |
Product condition:
The Lyophilized Amebocyte Lysate - LAL reagent sensitivity and the Control Standard Endotoxin potency are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte reagent kits come with product instruction, Certificate of Analysis, MSDS.
What is the difference between Bioendo single test vial and multiple test vial?
● Single test: reconstitute the single limulus lysate test or called limulus amebocyte by BET water in the glass vial or glass ampoule.
● Multi-test: reconstitute the lysate reagent with BET water, and then add marked amount of lysate reagent following COA to the reaction tube or well plate for use. There is no difference in the sample pre-processing procedure; according to the amount of testing used, the sample size used for a single test is larger than the sample size used for multiple tests.
Why the gel clot assay kit G52 special for mass samples quantity?
1. Multi test LAL reagent for endotoxin detection in the applications of mass samples’ LAL assay operation procedures.
2. G52 series of Gel clot endotoxin assay multi test glass vial no need sophisticated microplate reader. In LAL assay its procedure of incubation by water bath or dry heat incubator is convenient device.
3. High end quality of endotoxin free tube (<0.005EU/ml) and High quality of pyrogen free tips (<0.005EU/ml) as the guaranteed consumables to ensure the correct result.
4. To choose Bioendo single LAL test vial or multi LAL test vial by samples quantity, the target is LAL test for pyrogens detection.
Related products in the endotoxin test assay:
Water for Bacterial Endotoxins Test (BET), Recommend TRW50 or TRW100
Endotoxin free glass tube ( dilution tube ), Recommend T1310018 and T107540
Pyrogen free tips, Recommend PT25096 or PT100096
Pipettor, Recommend PSB0220
Test Tube Rack
Incubation Instrument (Water Bath or Dry Heat Incubator ), to recommend Bioendo Dry Heat Incubator TAL-M2 is 60 holes one modular.
Vortex Mixter, Recommend VXH.
Control Standard Endotoxin, CSE10V.