PriceList for Pyrogen Free Tube - Eight-channel Mechanical Pipette – Bioendo
PriceList for Pyrogen Free Tube - Eight-channel Mechanical Pipette – Bioendo Detail:
Eight-Channel Mechanical Pipettor
1. Product information
All multi-channel mechanical pipettor have been quality tested according to ISO8655-2:2002 with calibration certificate. The quality control involves gravimetric testing of each pipette with distilled water at 22℃. The multichannel mechanical pipettor is idea for the detectionof bacterial endotoxin lal endotoxin testing by kinetic turbidimetric andkinetic chromogenic method.
- Eight-Channel Mechanical Pipettor is available for standard 96-well plate
- Dispensing head rotates for optimum pipetting convenience
- Individual piston and tip cone assemblies allow easy repair and maintenance
- Compound material tip cone design allows visual seal verification
- Can be used with universal style pipette tips
-good for kinetic chromogenic, kinetic turbidimetric TAL orend-point chromogenic TAL endotoxin assay
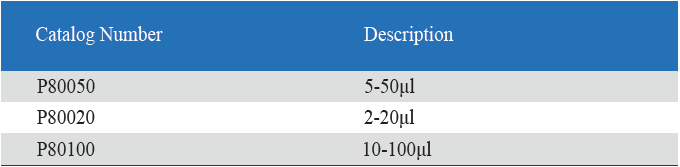
Product detail pictures:

Related Product Guide:
We retain bettering and perfecting our goods and service. At the same time, we perform actively to do research and enhancement for PriceList for Pyrogen Free Tube - Eight-channel Mechanical Pipette – Bioendo , The product will supply to all over the world, such as: Oman, Palestine, Nigeria, Besides strong technical strength, we also introduce advanced equipment for inspection and conduct strict management. All the staff of our company welcome friends both at home and abroad to come for visits and business on the basis of equality and mutual benefit. If you are interested in any of our items, please feel free to contact us for quotation and product details.
Problems can be quickly and effectively resolved, it is worth to be trust and working together.






