OEM/ODM Supplier Kit LAL - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo
OEM/ODM Supplier Kit LAL - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo Detail:
Single-Channel Mechanical Pipettes (Semi-sterile)
1. Product Information
The Single-Channel Mechanical Pipettes (Semi-sterile) is adjustable, which could be used to dispense liquid precisely. Measuring volume of our Single-Channel Mechanical Pipettes (Semi-sterile) ranges from 0.1μL to 5mL. Products are produced based on ISO8655/DIN12650. It could be widely employed on endotoxin detection, etc..
2. Product Features
- Light weight, economic
- The pipettes cover volume range from 0.1μL to 5mL
- Semi-sterile
- Easy calibration and maintenance
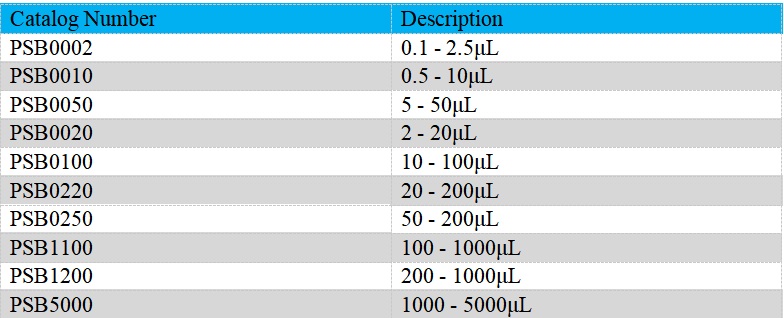
Product detail pictures:

Related Product Guide:
We support our buyers with ideal high quality products and high level service. Becoming the specialist manufacturer in this sector, we have gained rich practical experience in producing and managing for OEM/ODM Supplier Kit LAL - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo , The product will supply to all over the world, such as: Jeddah, Mecca, United Kingdom, We have now a good reputation for stable quality goods, well received by customers at home and abroad. Our company would be guided by the idea of "Standing in Domestic Markets, Walking into International Markets". We sincerely hope that we could do business with car manufacturers, auto part buyers and the majority of colleagues both at home and abroad. We expect sincere cooperation and common development!
We are long-term partners, there is no disappointment every time, we hope to maintain this friendship later!