OEM/ODM Factory Application Of LAL Test - Eight-channel Mechanical Pipette – Bioendo
OEM/ODM Factory Application Of LAL Test - Eight-channel Mechanical Pipette – Bioendo Detail:
Eight-Channel Mechanical Pipettor
1. Product information
All multi-channel mechanical pipettor have been quality tested according to ISO8655-2:2002 with calibration certificate. The quality control involves gravimetric testing of each pipette with distilled water at 22℃. The multichannel mechanical pipettor is idea for the detectionof bacterial endotoxin lal endotoxin testing by kinetic turbidimetric andkinetic chromogenic method.
- Eight-Channel Mechanical Pipettor is available for standard 96-well plate
- Dispensing head rotates for optimum pipetting convenience
- Individual piston and tip cone assemblies allow easy repair and maintenance
- Compound material tip cone design allows visual seal verification
- Can be used with universal style pipette tips
-good for kinetic chromogenic, kinetic turbidimetric TAL orend-point chromogenic TAL endotoxin assay
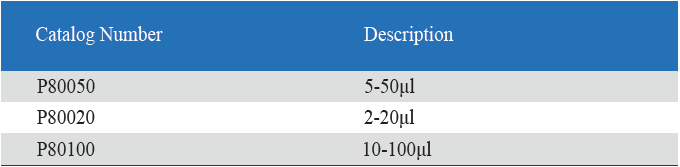
Product detail pictures:

Related Product Guide:
Our purpose is to fulfill our clients by offering golden company, great price and premium quality for OEM/ODM Factory Application Of LAL Test - Eight-channel Mechanical Pipette – Bioendo , The product will supply to all over the world, such as: Uruguay, Auckland, Sheffield, We hope to have long-term cooperation relationships with our clients. If you are interested in any of our products, make sure you do not hesitate to send enquiry to us/company name. We ensure that you can be totally satisfied with our best solutions!
As a veteran of this industry, we can say that the company can be a leader in the industry, select them is right.