New Fashion Design for Bacterial Endotoxin Test Method Validation Protocol - Depyrogenated Sample Bottles ( Endotoxin Free ) – Bioendo
New Fashion Design for Bacterial Endotoxin Test Method Validation Protocol - Depyrogenated Sample Bottles ( Endotoxin Free ) – Bioendo Detail:
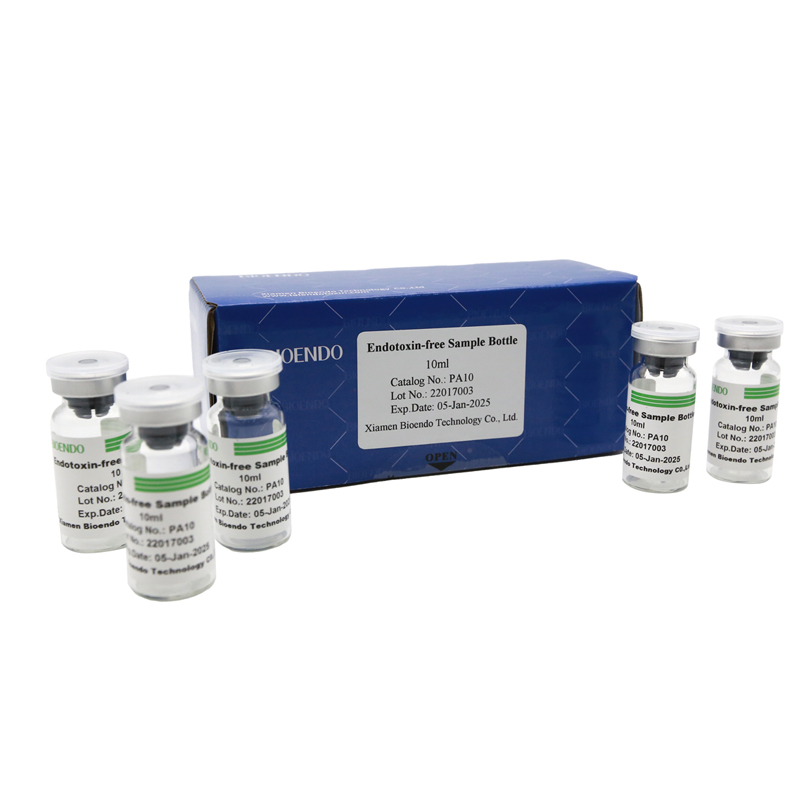
Depyrogenated Sample Container
1. Product information
We offer various of low endotoxin, pyrogen free accessories products,includes Water for Bacterial Endotoxins Test, pyrogen-free test tubes, pyrogen-free pipettor tips, microplates and sample bottles for your conveniences. High quality depyrogenated low endotoxin pyrogen free products insure the success of your experiments.
Depyrogenated (Endotoxin Free) Sample container(endotoxin free bottle, pyrogen free bottle, pyrogen free sample bottle)are glass bottles contain less than 0.005 EU/ml endotoxin. These bottles could be used to store various samples for lps endotoxin test, such as protein solution, vaccines, DNA solutions, dialysate, water for injections, etc., for endotoxin testing. Come with endotoxin free seals.
2. Product parameter
Endotoxin level < 0.005 EU/ml
3. Product features and application
For preparing and storage of the test samples.
| Catalog Number | Descriptions | Package |
| PA2 | Endotoxin free sample glass ampoule, 2ml | 10Pcs/Pack |
| PA10 | Endotoxin free sample glass vial, 10ml | 10Pcs/Pack or 110Pcs/Pack |
| PA50 | Endotoxin free sample glass vial, 50ml | 10Pcs/Pack |
| PA125 | Pyrogen free sample bottle, 125ml | 1Pcs/Pack |
| PA500 | Pyrogen free sample bottle, 500ml | 1Pcs/Pack |
such as endotoxin free tubes; endotoxin free tips; endotoxin free microplates; endotoxin free sample bottles;
According to China Pharmacopoeia, the utensils needed in the procedure of endotoxin test assay, such as sample vessel, dilution and reaction tubes, pipette tips, have to choose endotoxin free consumables. the utensils needed for the experiment need to be processed to remove possible exogenous endotoxins. If the endotoxin is not removed, it will interfere with the experiment.
Product detail pictures:





Related Product Guide:
Our company has been concentrating on brand strategy. Customers' pleasure is our greatest advertising. We also source OEM service for New Fashion Design for Bacterial Endotoxin Test Method Validation Protocol - Depyrogenated Sample Bottles ( Endotoxin Free ) – Bioendo , The product will supply to all over the world, such as: Morocco, UK, Israel, To achieve reciprocal advantages, our company is widely boosting our tactics of globalization in terms of communication with overseas customers, fast delivery, the best quality and long-term cooperation. Our company upholds the spirit of "innovation, harmony, team work and sharing, trails, pragmatic progress". Give us a chance and we will prove our capability. With your kind help, we believe that we can create a bright future with you together.
As an international trading company, we have numerous partners, but about your company, I just want to say, you are really good, wide range, good quality, reasonable prices, warm and thoughtful service, advanced technology and equipment and workers have professional training, feedback and product update is timely, in short, this is a very pleasant cooperation, and we look forward to the next cooperation!







