Manufacturer of Dry Heat Sterilization Validation – Mini Dry Heat Incubator – Bioendo
Manufacturer of Dry Heat Sterilization Validation – Mini Dry Heat Incubator – Bioendo Detail:
Dry heat incubator single module
1. Product information
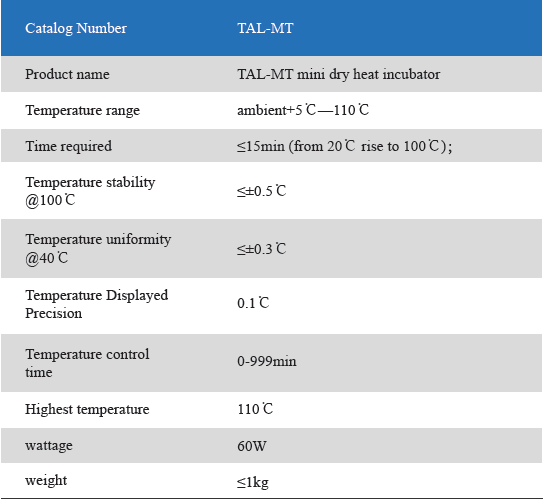
The Mini Dry Heat Incubator is a micro-processor controlled heating block with semi conductor heating technology.It adapts onboard use, smart, light and convenient for movement, suit for any kind of occasions. Especially good for the incubation of the gel clot LAL assay, LAL chromogenic endpoint assay incubation.
2. Product features
1. Unique designed. Smart and light, convenient movement, suit for various occasions.
2. LCD simultaneously display setting and actual time and temperature.Temperature calibration function.
3. Automatic fault detection function with buzzer alarm.
4. 24V DC input power, built-in over-temperature protection device.
5. Various of blocks for optional choice. Convenient for replacement. Easy cleaning and disinfection.
Product detail pictures:



Related Product Guide:
We thinks what buyers think, the urgency of urgency to act during the interests of a purchaser position of theory, allowing for much better high-quality, reduced processing costs, charges are more reasonable, won the new and outdated consumers the support and affirmation for Manufacturer of Dry Heat Sterilization Validation – Mini Dry Heat Incubator – Bioendo , The product will supply to all over the world, such as: Oman, Melbourne, Afghanistan, As a way to make use of the resource on the expanding information and facts in international trade, we welcome prospects from everywhere on the web and offline. In spite in the top quality products we offer, effective and satisfying consultation service is supplied by our specialist after-sale service group. Solution lists and detailed parameters and any other info weil be sent to you timely for the inquiries. So please get in touch with us by sending us emails or contact us if you have any concerns about our firm. ou can also get our address info from our web site and come to our enterprise. or a field survey of our solutions. We're confident that we are going to share mutual results and build solid co-operation relations with our companions in this market. We're looking forward to your inquiries.
Sales manager is very enthusiastic and professional, gave us a great concessions and product quality is very good,thank you very much!







