Manufacturer of Dry Heat Sterilization Validation – Mini Dry Heat Incubator – Bioendo
Manufacturer of Dry Heat Sterilization Validation – Mini Dry Heat Incubator – Bioendo Detail:
Dry heat incubator single module
1. Product information
The Mini Dry Heat Incubator is a micro-processor controlled heating block with semi conductor heating technology.It adapts onboard use, smart, light and convenient for movement, suit for any kind of occasions. Especially good for the incubation of the gel clot LAL assay, LAL chromogenic endpoint assay incubation.
2. Product features
1. Unique designed. Smart and light, convenient movement, suit for various occasions.
2. LCD simultaneously display setting and actual time and temperature.Temperature calibration function.
3. Automatic fault detection function with buzzer alarm.
4. 24V DC input power, built-in over-temperature protection device.
5. Various of blocks for optional choice. Convenient for replacement. Easy cleaning and disinfection.
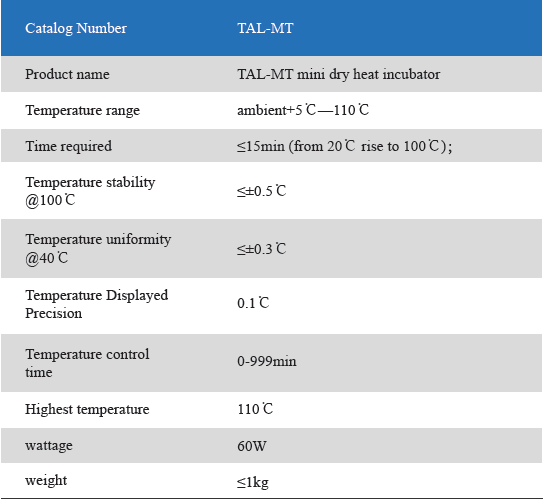
Product detail pictures:



Related Product Guide:
abide by the contract", conforms on the market requirement, joins from the market competition by its good quality likewise as provides more comprehensive and superb support for customers to let them become large winner. The pursue of the company, is definitely the clients' pleasure for Manufacturer of Dry Heat Sterilization Validation – Mini Dry Heat Incubator – Bioendo , The product will supply to all over the world, such as: luzern, Tunisia, Anguilla, We always adhere to follow the honesty, mutual benefit, common development, after years of development and the tireless efforts of all staff, now has perfect export system, diversified logistics solutions, thorough meet customer shipping, air transport, international express and logistics services. Elaborate one-stop sourcing platform for our customers!
The company comply with the contract strict, a very reputable manufacturers, worthy a long-term cooperation.