Hot-selling LAL Turbidimetric test - Bioendo KT Endotoxin Test Kit (Kinetic Turbidimetric Assay) – Bioendo
Hot-selling LAL Turbidimetric test - Bioendo KT Endotoxin Test Kit (Kinetic Turbidimetric Assay) – Bioendo Detail:
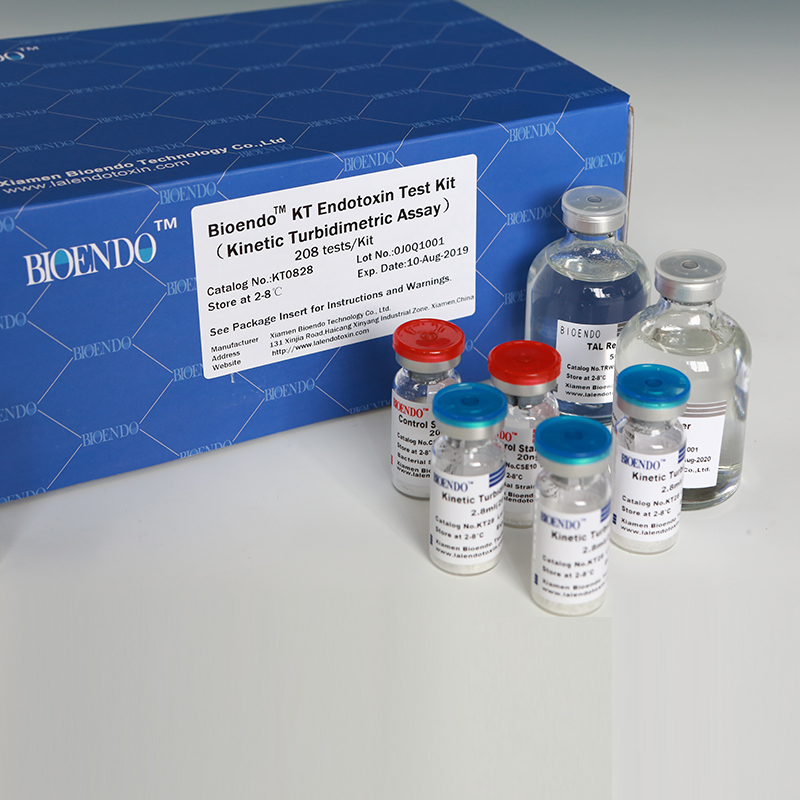
Bioendo KT Endotoxin Test Kit (Kinetic Turbidimetric Assay)
1. Product Introduction
Kinetic Turbidimetric Amebocyte Lysate Vial is developed based on the principle that the time needed to reach a certain absorbance increase (onset OD), i.e. onset time, is negatively correlated with the endotoxin concentration. Sensitivity could reach 0.005EU/ml, and the detection could reach four orders of magnitude. It is specially suitable for pharmaceuticals industry to monitor endotoxin concentration.
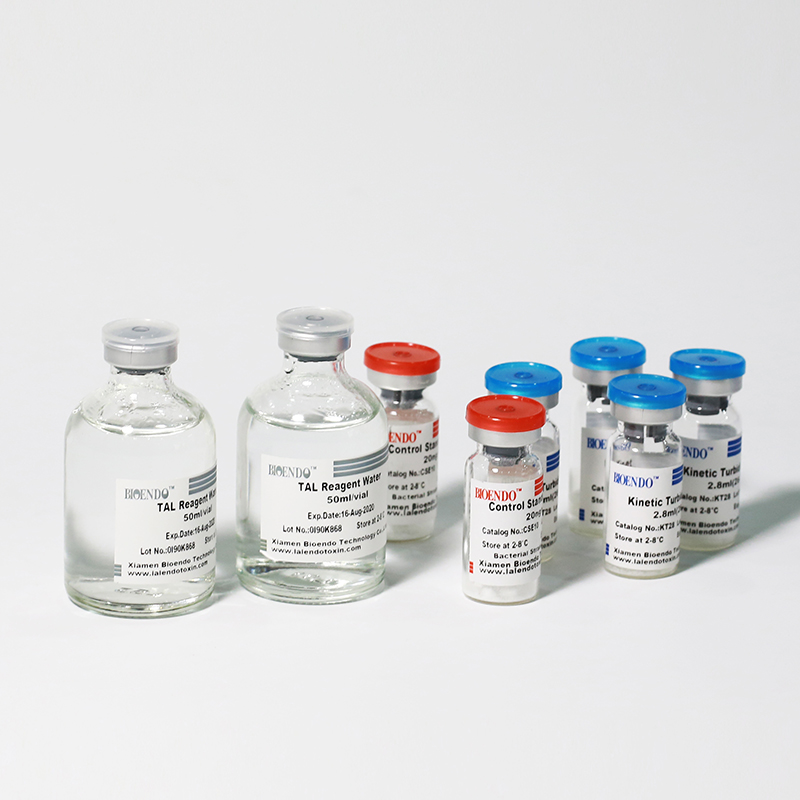
The kit contains Lyophilized Amebocyte Lysate, Control Standard Endotoxin, and Water for BET. KT Endotoxin Test Kit (Kinetic Turbidimetric Assay) require a kinetic microplate reader such as ELx808IULALXH or a kinetic tube reader. Kinetic software is also required for calculation of the endotoxin concentration.
2. Product Parameter
Assay Range: 0.005 – 5EU/ml; 0.01 – 10EU/ml
3. Product Application
End-product endotoxin (pyrogen) qualification, Waterfor injection endotoxin assay, raw material endotoxin testing or endotoxinlevel monitoring during manufacturing process for pharmaceutical companies ormedical devices manufacturers.
Note:
Lyophilized Amebocyte Lysate reagent manufactured by Bioendo is made from amebocyte lysate from the horseshoe crab (Tachypleus tridentatus).
|
Catalog No. |
Description |
Kit Contents |
Sensitivity EU/ml |
|
KT0817 |
Bioendo™ KT Endotoxin Test Kit (Kinetic Turbidimetric Assay), 128 tests/kit |
8 Lyophilized Amebocyte Lysate, 1.7ml (16 Tests/vial); 8 Reconstitution Buffer, 3.0ml/vial; 4 CSE; 2 Water for BET, 50ml/vial; |
0.01-10EU/ml |
|
KT0817S |
0.005-5EU/ml, 0.01-10EU/ml |
||
|
KT0852 |
Bioendo™ KT Endotoxin Test Kit (Kinetic Turbidimetric Assay), 400 Tests/Kit |
8 Lyophilized Amebocyte Lysate, 5.2ml (50 Tests/Vial); 8 Reconstitution Buffer, 6.0ml/vial; 4 CSE; 3 Water for BET, 50ml/vial; |
0.01-10EU/ml |
|
KT0852S |
0.005-5EU/ml, 0.01-10EU/ml |
||
|
KT5017 |
Bioendo™ KT Endotoxin Test Kit (Kinetic Turbidimetric Assay), 800 Tests/Kit |
50 Lyophilized Amebocyte Lysate, 1.7ml (16 Tests/vial); 50 Reconstitution Buffer, 3.0ml/vial; 10 CSE; |
0.01-10EU/ml |
|
KT5017S |
0.005-5EU/ml, 0.01-10EU/ml |
||
|
KT5052 |
Bioendo™ KT Endotoxin Test Kit (Kinetic Turbidimetric Assay), 2500 Tests/Kit |
50 Lyophilized Amebocyte Lysate,5.2ml (50 Tests/vial); 50 Reconstitution Buffer, 6.0ml/vial; 10 CSE; |
0.01-10EU/ml |
|
KT5052S |
0.005-5EU/ml, 0.01-10EU/ml |
Product Condition:
The potency of Lyophilized Amebocyte Lysate and Control Standard Endotoxin are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come with product instruction, Certificate of Analysis, MSDS.
Product detail pictures:



Related Product Guide:
We normally think and practice corresponding towards the change of circumstance, and grow up. We aim at the achievement of a richer mind and body plus the living for Hot-selling LAL Turbidimetric test - Bioendo KT Endotoxin Test Kit (Kinetic Turbidimetric Assay) – Bioendo , The product will supply to all over the world, such as: Puerto Rico, United States, Swiss, During the short years, we serve our clients honestly as Quality First, Integrity Prime, Delivery Timely, which has earned us an outstanding reputation and an impressive client care portfolio. Looking forward to working with you Now!
Adhering to the business principle of mutual benefits, we have a happy and successful transaction, we think we will be the best business partner.