Good Quality LAL Test - Mini Dry Heat Incubator – Bioendo
Good Quality LAL Test - Mini Dry Heat Incubator – Bioendo Detail:
Dry heat incubator single module
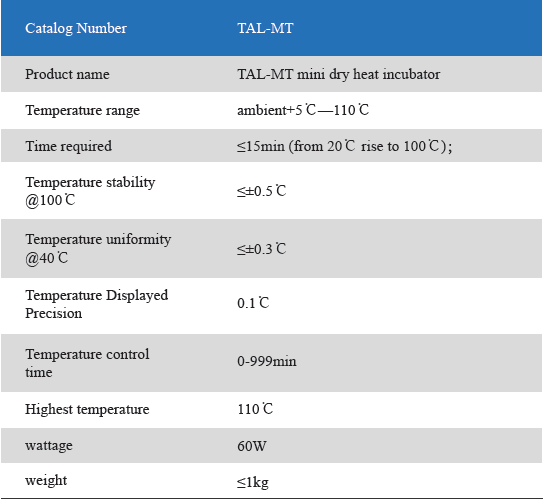
1. Product information
The Mini Dry Heat Incubator is a micro-processor controlled heating block with semi conductor heating technology.It adapts onboard use, smart, light and convenient for movement, suit for any kind of occasions. Especially good for the incubation of the gel clot LAL assay, LAL chromogenic endpoint assay incubation.
2. Product features
1. Unique designed. Smart and light, convenient movement, suit for various occasions.
2. LCD simultaneously display setting and actual time and temperature.Temperature calibration function.
3. Automatic fault detection function with buzzer alarm.
4. 24V DC input power, built-in over-temperature protection device.
5. Various of blocks for optional choice. Convenient for replacement. Easy cleaning and disinfection.
Product detail pictures:



Related Product Guide:
We're going to commit ourselves to giving our esteemed customers along with the most enthusiastically considerate providers for Good Quality LAL Test - Mini Dry Heat Incubator – Bioendo , The product will supply to all over the world, such as: Belgium, France, Johannesburg, We pay high attention to customer service, and cherish every customer. We've maintained a strong reputation in the industry for many years. We are honest and work on building a long-term relationship with our customers.
The company can think what our think, the urgency of urgency to act in the interests of our position, can be said this is a responsible company, we had a happy cooperation!