Factory For Bacterial Endotoxin Test Usp Chapter - Control Standard Endotoxin (CSE) – Bioendo
Factory For Bacterial Endotoxin Test Usp Chapter - Control Standard Endotoxin (CSE) – Bioendo Detail:
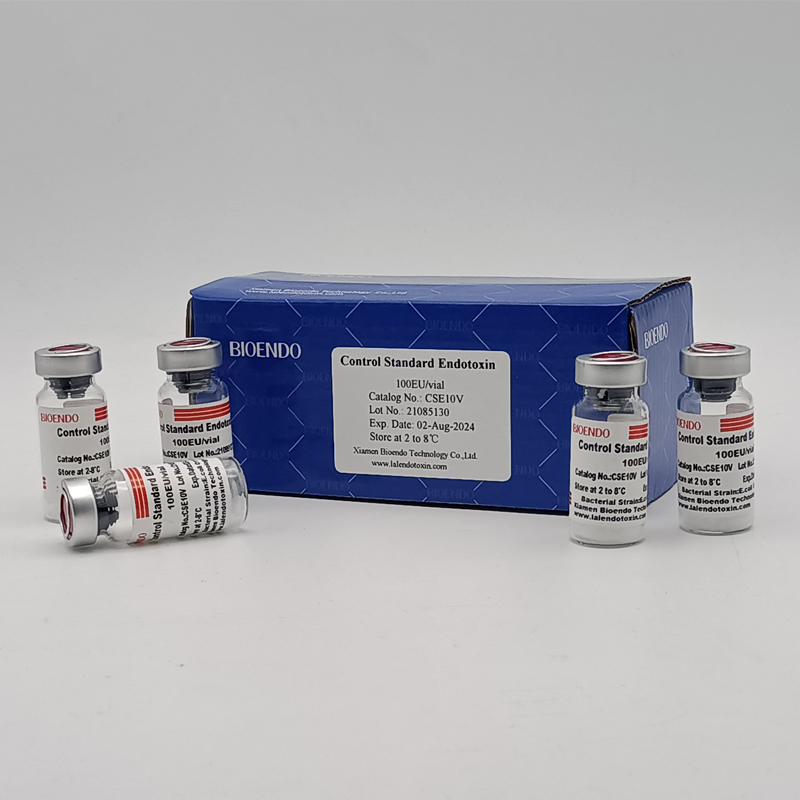
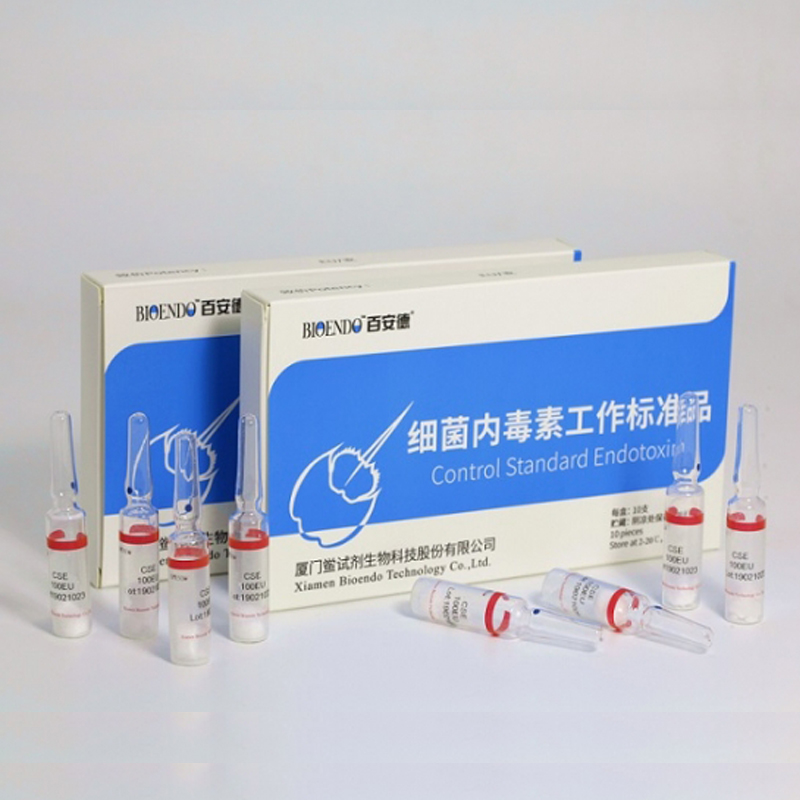
Control Standard Endotoxin (CSE)
1. Product Information
Control Standard Endotoxin (CSE) is extracted from E.coli O111:B4. CSE is an economic alternative to Reference Standard Endotoxin (RSE) in constructing standard curves, validating product and preparing controls in Lyophilized Amebocyte Lysate test. The labeled potency of CSE endotoxinE.coli standard is referenced against RSE. The Control Standard Endotoxin could be used with gel clot assay, kinetic turbidimetric assay or kinetic chromogenic assay as the endotoxin testing standards. The Certificate of Analysis will show the matched Lyophilized Amebocyte Lysate reagent lots.
2. Product Parameter
| Catalog Number | Potency (EU/vial) | Package |
| CSE10V | 100 to 999 EU | seal in glass vial, 10vials/pack |
| CSE100V | 1 to 199 EU | seal in glass vial, 10vials/pack |
| CSE10A | 1 to 99 EU | seal in glass ampoule, 10vials/pack |
3. Product Feature and Application
Bioendo CSE was labeled by the potency and matched to Lyophilized Amebocyte Lysate reagent lots. Users do not need to do the CSE/RSE ratio assay. Low potency control standard endotoxin is available to avoid lots of steps of dilution to provide convenience for end users.
Product Condition:
Control Standard Endotoxin (CSE), extracted from E.coli O111:B4, is an economic alternative to Reference Standard Endotoxin (RSE) in constructing standard curves, validating product and preparing controls in endotoxin test. The potency of CSE is referenced against USP Reference Standard Endotoxin, and labeled in the Certificate of Analysis.
Endotoxin test assay: Lysate reagent and CSE lot number have to be matched.
Pyrogen free tip box
Endotoxin free tubes
Product detail pictures:


Related Product Guide:
We know that we only thrive if we can guarantee our combined rate competiveness and good quality advantageous at the same time for Factory For Bacterial Endotoxin Test Usp Chapter - Control Standard Endotoxin (CSE) – Bioendo , The product will supply to all over the world, such as: Australia, Kenya, Australia, So far our merchandise have been exported to east Europe, the Middle East, Southeast, Africa and South America etc. We have now 13years experienced sales and purchase in Isuzu parts at home and abroad and the ownership of the modernized electronic Isuzu parts checking systems. We honor our core principal of Honesty in business, priority in service and will do our best to provide our customers with high quality items and excellent service.
Sales manager is very enthusiastic and professional, gave us a great concessions and product quality is very good,thank you very much!