Chinese wholesale Chromogenic endotoxin assay - Micro Kinetic Chromogenic Endotoxin Assay Kit – Bioendo
Chinese wholesale Chromogenic endotoxin assay - Micro Kinetic Chromogenic Endotoxin Assay Kit – Bioendo Detail:
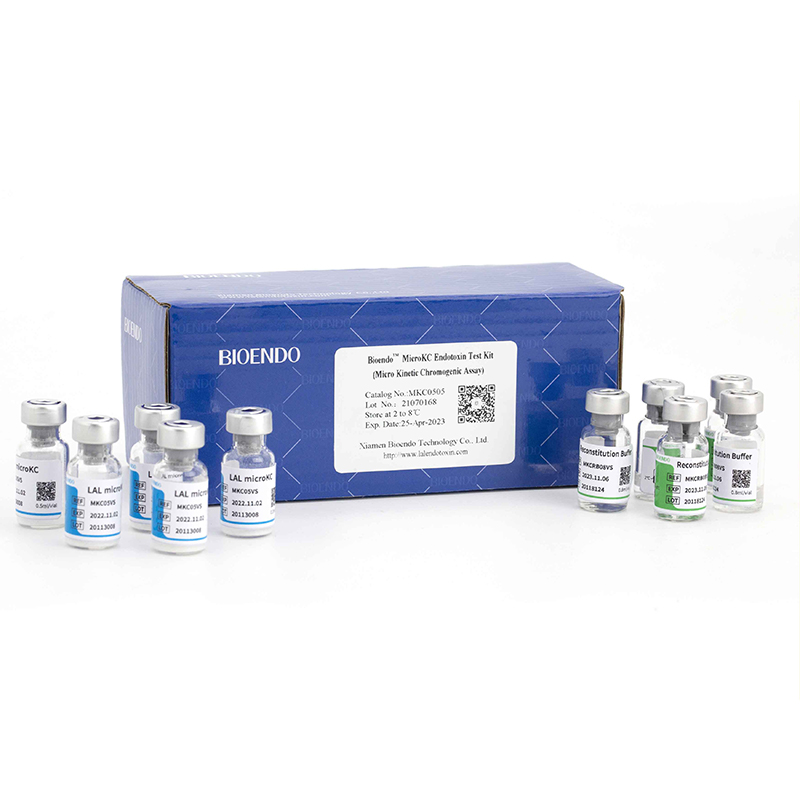
Micro KC Endotoxin Test Kit (Micro Kinetic Chromogenic Endotoxin Assay)
The Bioendo MicroKC Endotoxin Test Kit (Micro Kinetic Chromogenic Endotoxin Assay), which adopts the micro-technical kinetic chromogenic method, is equipped with a special customized 96-well microplates, which can be used in the endotoxin test of microbial detection system Elx808IULALXH and other conventional endotoxin detection equipment to realize the micro-kinetic chromogenic endotoxin detection. The micro KC kit meets the requirements of the rules of Bacterial Endotoxin Test in Pharmacopoeia. Each test requires only the 25μL sample for test and 25μL of lysate reagent. The testing principle is the same as that of the traditional kinetic chromogenic method for endotoxin detection. It has strong anti-interference ability and is very suitable for endotoxins quantitative detection in parenteral drugs, vaccines, antibodies, biological preparations and so on.
Typical users:
Pharmaceutical manufacturers, medical devices manufacturers, scientific research institutions.
Ø Less dosage of sample for test , and each test only needs 25μL of samples for testing.
Ø Adopting the pharmacopoeia-approved kinetic chromogenic method technology which can meet the requirements of GMP for data integrity and traceability.
Ø High sensitivity, up to 0.005EU/ml.
Ø Strong anti-interference ability, does not require coagulogen to form a gel, and accurately quantifies bacterial endotoxins based on the color reaction.
Ø Easy to operate, complete 96 tests at one time, and the system automatically detects and analyzes in one step.
Ø High cost-effective, higher performance.
Ø Supporting 8-well plate for micro-detection
Technical Parameters:
|
Catalog No. |
Description |
Kit Contents |
Sensitivity EU/ml |
||
|
MKC0505VS |
Bioendo™ KC Endotoxin Test Kit (Micro Kinetic Chromogenic Assay), 90 Tests/Kit |
5 Chromogenic Amebocyte Lysate, 0.5ml (18 Tests/Vial); 5 Reconstitution Buffer, 2.0ml/vial; |
0.005 to 5EU/ml |
||
|
MKC0505V |
0.01 to 10EU/ml |
||||
|
MKC0505AS |
Bioendo™ KC Endotoxin Test Kit (Micro Kinetic Chromogenic Assay), 90 Tests/Kit |
5 Chromogenic Amebocyte Lysate, 0.5ml (18 Tests/ampoule); 5 Reconstitution Buffer, 2.0ml/vial; |
0.005 to 5EU/ml |
||
|
MKC0505A |
0.01 to 10EU/ml |
||||
|
MPMC96 |
8 wells per strip |
96 wells per microplate, 12pcs detachable strips. |
|||
What is the main feature of new Bioendo microKC kit?
Each test requires only the 25μL sample for test and 25μL of lysate reagent. Provide the professional solution that is very friendly in terms of resource utilization.
Product detail pictures:



Related Product Guide:
We're commitment to offer you the competitive price ,remarkable products excellent, also as fast delivery for Chinese wholesale Chromogenic endotoxin assay - Micro Kinetic Chromogenic Endotoxin Assay Kit – Bioendo , The product will supply to all over the world, such as: Miami, Puerto Rico, Durban, All these products are manufactured in our factory located in China. So we can guarantee our quality seriously and availably. Within these four years we sell not only our products but also our service to clients throughout the world.
This company conforms to the market requirement and joins in the market competition by its high quality product, this is an enterprise that have Chinese spirit.








