Best quality LAL Reagent Water - Rapid Gel Clot single test kit – Bioendo
Best quality LAL Reagent Water - Rapid Gel Clot single test kit – Bioendo Detail:
Rapid Gel Clot Endotoxin Test Kit (Single Sample Test Kit)
1. Product Information
Rapid Gel Clot Endotoxin Assay Kit is designed to rapidly quantify endotoxin in water or dialysate. Generally, read the result in about 30 minutes.
Under the guidance of detecting endotoxin in water or dialysate quickly, endotoxin detection with Bioendo Rapid Gel Clot Endotoxin Assay Kit does not need to multi steps’ dilution of Control Standard Endotoxin and test samples. Easy and convenient procedures in the operation of rapid endotoxin assay, the whole operation not required sophisticated experimental equipment, incubation by a dry heat incubator. It is especial suitable for the endotoxins analysis in the water or dialysate.
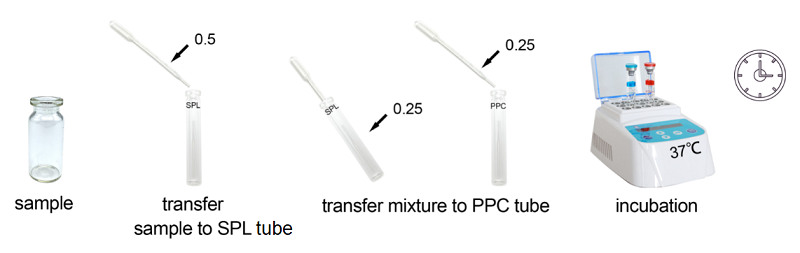
2. Product Parameter
Sensitivity Range: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25EU/ml, 0.5EU/ml
One sample test in a kit.
Assay time: less than 30 minutes.
|
Catalog No. |
Description |
Kit Contents |
Sensitivity EU/ml |
Reaction Time minutes |
|
RG025003 |
BioendoTM Rapid Gel Clot Endotoxin Assay Kit, One Sample Kit |
1 SPL Tube; 1 PPC Tube; 1 Endotoxin-free Sample Bottle; 3 Pyrogen-free Pipettors; |
0.03 |
≤60 |
|
RG025006 |
0.06 |
≤60 |
||
|
RG0250125 |
0.125 |
≤45 |
||
|
RG025025 |
0.25 |
≤30 |
||
|
RG025050 |
0.5 |
≤30 |
3. Kit Application
Bioendo single test Rapid Gel Clot Endotoxin Assay Kit provide a kind of rapid endotoxin testing solution, the featured application in the field of dialysis.
Note:
Lyophilized Amebocyte Lysate (LAL) reagent manufactured by Bioendo is made from amebocyte lysate derived blood of horseshoe crab. In the operation procedure, the dilution of control standard endotoxin is simple and convenient. Endotoxin free sample container and pyrogen free transfer pipette is required, Sophisticated instrument is not required, recommend Bioendo Dry heat incubator TAL-MT use in the procedure of incubation.
Kit Configuration:
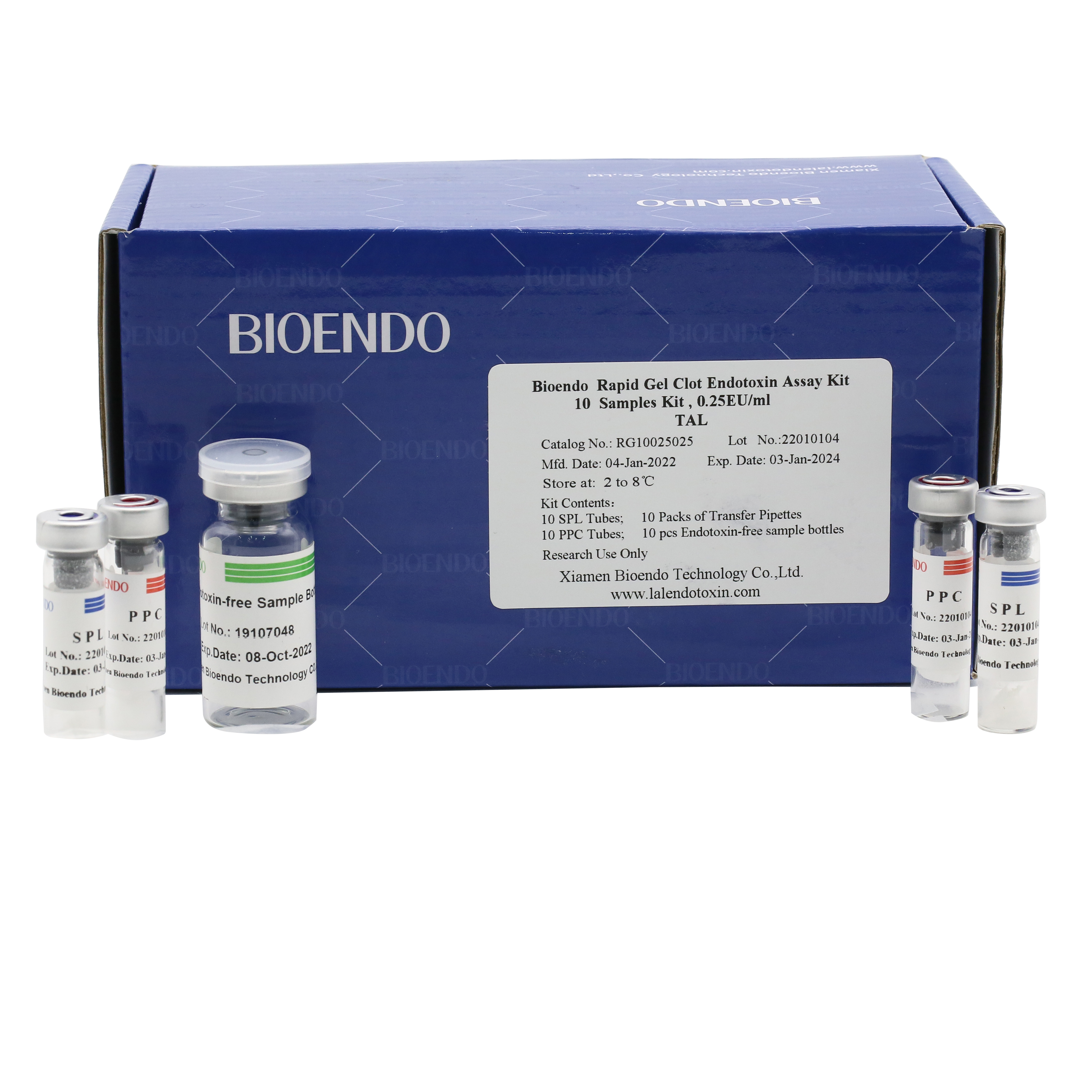
Bioendo single test Rapid Gel Clot Endotoxin Assay Kit contains:
1 piece of SPL Tube, 1 piece of PPC Tube, 1 piece of Endotoxin-free Sample Bottle. (endotoxin free top level),
1 Pack of Transfer Pipette 3 pieces. (endotoxin free top level)
Product detail pictures:



Related Product Guide:
As a way to ideal meet up with client's desires, all of our operations are strictly performed in line with our motto "High Top quality, Competitive Cost, Fast Service" for Best quality LAL Reagent Water - Rapid Gel Clot single test kit – Bioendo , The product will supply to all over the world, such as: Nepal, Belgium, Chicago, They are sturdy modeling and promoting effectively all over the world. Never ever disappearing major functions within a quick time, it's a have to in your case of fantastic good quality. Guided by the principle of "Prudence, Efficiency, Union and Innovation. the corporation. ake an excellent efforts to expand its international trade, raise its organization. rofit and raise its export scale. We have been confident that we've been going to have a bright prospect and to be distributed all over the world in the years to come.
In our cooperated wholesalers, this company has the best quality and reasonable price, they are our first choice.








