2022 wholesale price Kinetic Turbidimetric vs Kinetic Chromogenic - Bioendo KC Endotoxin Test Kit (Kinetic Chromogenic Assay) – Bioendo
2022 wholesale price Kinetic Turbidimetric vs Kinetic Chromogenic - Bioendo KC Endotoxin Test Kit (Kinetic Chromogenic Assay) – Bioendo Detail:
Bioendo KC Endotoxin Test Kit (Kinetic Chromogenic Assay)
1. Product Information
In Bioendo KC Endotoxin Test Kit, Amebocyte Lysate is co-lyophilized with chromogenic substrate. Therefore, bacterial endotoxin could be quantified based on the chromogenic reaction. The assay is strong resistance to interference, and has advantages of kinetic turbidimetric and end-point chromogenic method. Bioendo Endotoxin Test Kit contains Chromogenic Amebocyte Lysate, Reconstitution Buffer, CSE, Water for BET. Endotoxin detection with Kinetic Chromogenic method requires a kinetic incubating microplate reader such as ELx808IULALXH.
2. Product Parameter
Assay Range: 0.005 – 50EU/ml; 0.001 – 10EU/ml
|
Catalog No. |
Description |
Kit Contents |
Sensitivity EU/ml |
|
KC5028 |
Bioendo™ KC Endotoxin Test Kit (Kinetic Chromogenic Assay), 1300 Tests/Kit |
50 Chromogenic Amebocyte Lysate, 2.8ml (26 Tests/Vial); 50 Reconstitution Buffer, 3.0ml/vial; 10CSE; |
0.005-5EU/ml |
|
KC5028S |
0.001-10EU/ml |
||
|
KC0828 |
Bioendo™ KC Endotoxin Test Kit (Kinetic Chromogenic Assay), 208 Tests/Kit |
8 Chromogenic Amebocyte Lysate, 2.8ml (26 Tests/Vial); 8 Reconstitution Buffer, 3.0ml/vial; 4 CSE; 2 Water for BET, 50ml/vial; |
0.005-5EU/ml |
|
KC0828S |
0.001-10EU/ml |
||
|
KC5017 |
Bioendo™ KC Endotoxin Test Kit (Kinetic Chromogenic Assay), 800 Tests/Kit |
50 Chromogenic Amebocyte Lysate, 1.7ml (16 Tests/Vial); 50 Reconstitution Buffer, 2.0ml/vial; 10CSE; |
0.005-5 EU/ml |
|
KC5017S |
0.001-10 EU/m |
||
|
KC0817 |
Bioendo™ KC Endotoxin Test Kit (Kinetic Chromogenic Assay), 128 Tests/kit |
8 Kinetic Chromogenic Amebocyte Lysate, 1.7ml (16 Tests/vial); 8 Reconstitution Buffer, 2.0ml/vial; 4 CSE; 2 Water for BET, 50ml/vial; |
0.005-5 EU/ml |
|
KC0817S |
0.001-10 EU/ml |
3. Product Feature and Application
BioendoTM KC Endotoxin Test Kit (Kinetic Chromogenic Assay) features strong resistance to interference, and has advantages of kinetic turbidimetric and end-point chromogenic method. It is especially suitable for endotoxin detection of biological samples like vaccine, antibody, protein, nucleic acid, etc.
Note:
Lyophilized Amebocyte Lysate reagent manufactured by Bioendo is made from amebocyte lysate from the horseshoe crab (Tachypleus tridentatus).
Product Condition:
The sensitivity of Lyophilized Amebocyte Lysate and potency of Control Standard Endotoxin are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come with product instruction, Certificate of Analysis.
The kinetic chromogenic endotoxin test kit have to choose the microplate reader with 405nm filters.
Product detail pictures:

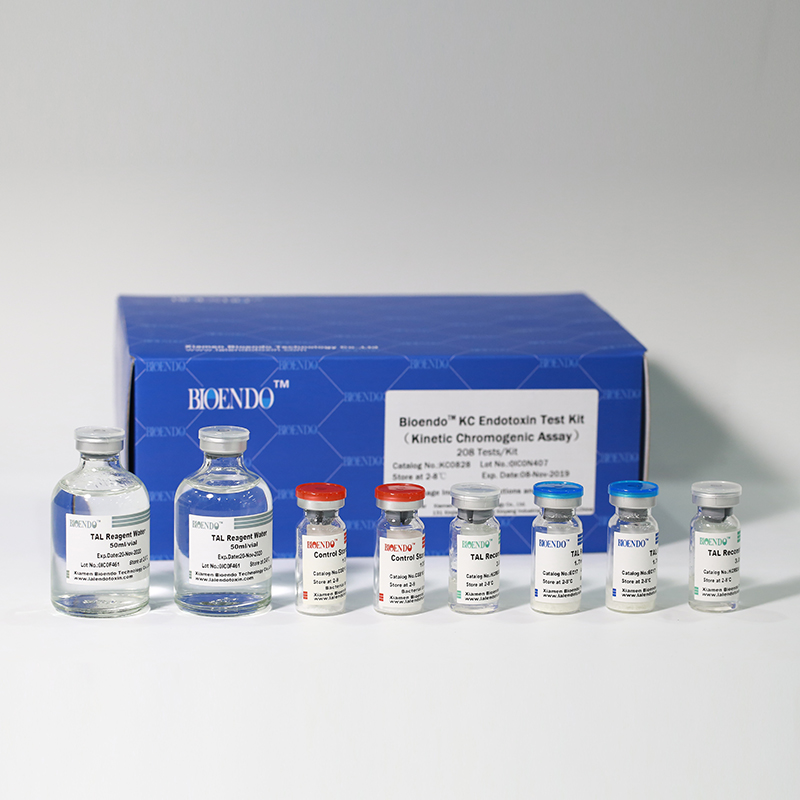


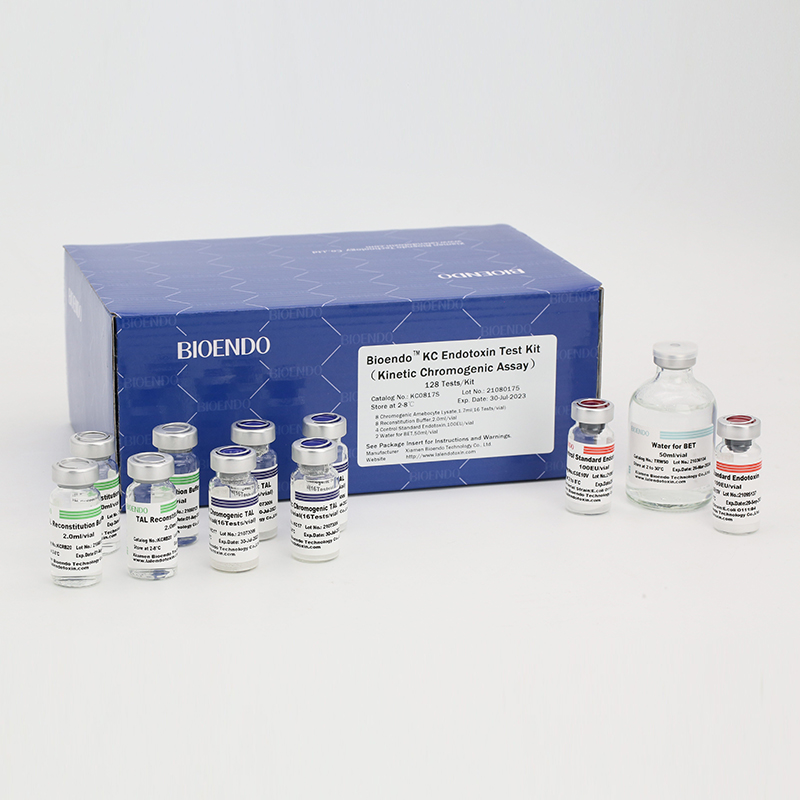
Related Product Guide:
We normally think and practice corresponding on the change of circumstance, and grow up. We aim at the achievement of a richer mind and body and also the living for 2022 wholesale price Kinetic Turbidimetric vs Kinetic Chromogenic - Bioendo KC Endotoxin Test Kit (Kinetic Chromogenic Assay) – Bioendo , The product will supply to all over the world, such as: Ecuador, United Arab emirates, Borussia Dortmund, We solution have passed through the national skilled certification and been well received in our key industry. Our specialist engineering team will often be ready to serve you for consultation and feedback. We've been able to also provide you with no cost samples to meet your needs. Best efforts are going to be produced to supply you the very best service and solutions. For anyone who is considering our business and solutions, please speak to us by sending us emails or get in touch with us right away. As a way to know our items and enterprise. lot more, you'll be able to come to our factory to find out it. We'll constantly welcome guests from around the globe to our firm. o build enterprise. elations with us. You should really feel absolutely free to make contact with us for small business and we believe we'll share the top trading practical experience with all our merchants.
We have been looking for a professional and responsible supplier, and now we find it.







