2022 China New Design LAL test for endotoxin - Control Standard Endotoxin (CSE) – Bioendo
2022 China New Design LAL test for endotoxin - Control Standard Endotoxin (CSE) – Bioendo Detail:
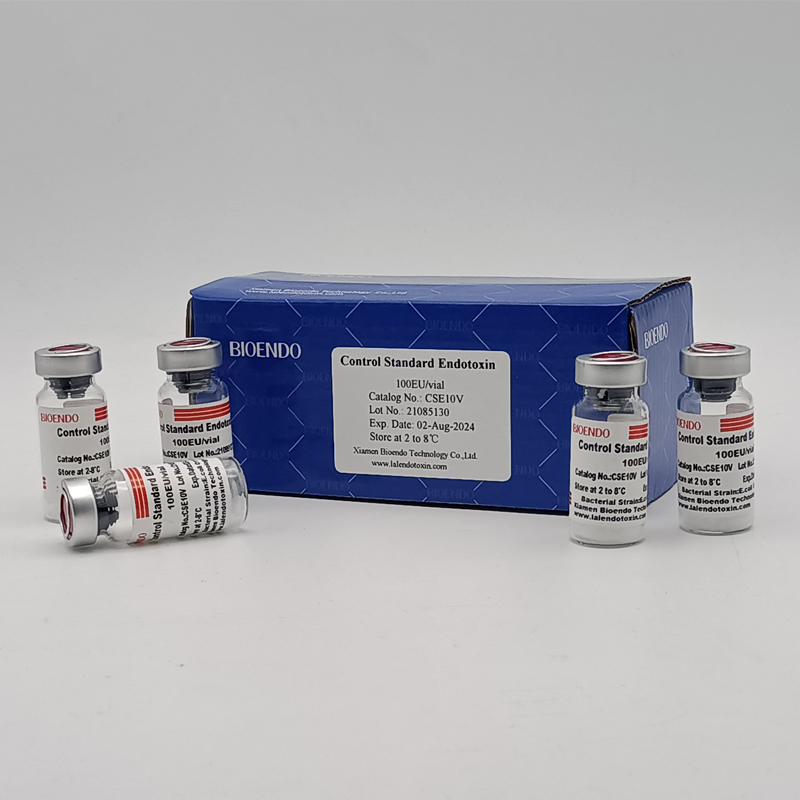
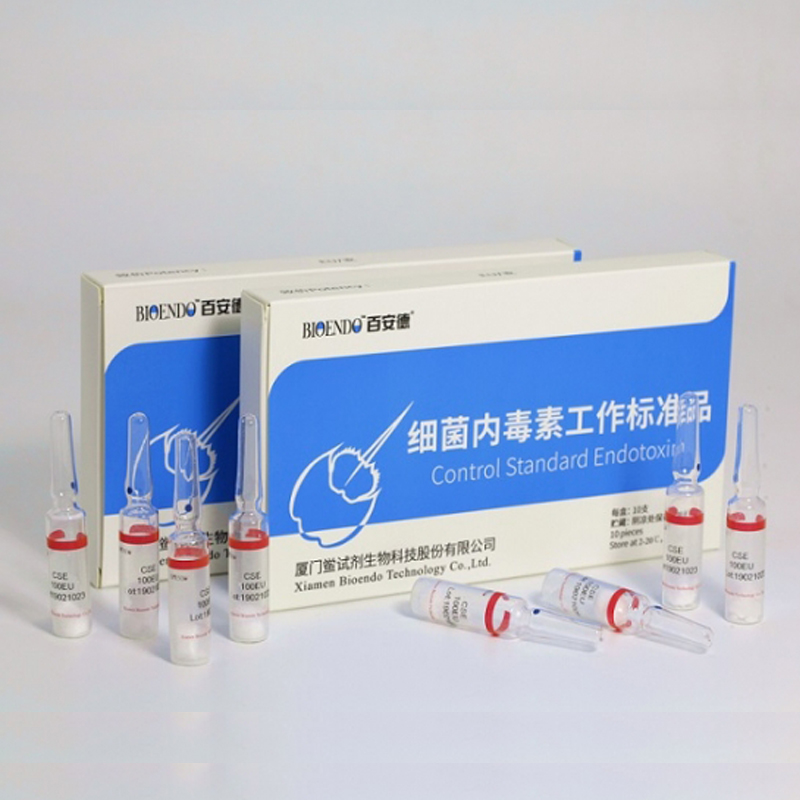
Control Standard Endotoxin (CSE)
1. Product Information
Control Standard Endotoxin (CSE) is extracted from E.coli O111:B4. CSE is an economic alternative to Reference Standard Endotoxin (RSE) in constructing standard curves, validating product and preparing controls in Lyophilized Amebocyte Lysate test. The labeled potency of CSE endotoxinE.coli standard is referenced against RSE. The Control Standard Endotoxin could be used with gel clot assay, kinetic turbidimetric assay or kinetic chromogenic assay as the endotoxin testing standards. The Certificate of Analysis will show the matched Lyophilized Amebocyte Lysate reagent lots.
2. Product Parameter
| Catalog Number | Potency (EU/vial) | Package |
| CSE10V | 100 to 999 EU | seal in glass vial, 10vials/pack |
| CSE100V | 1 to 199 EU | seal in glass vial, 10vials/pack |
| CSE10A | 1 to 99 EU | seal in glass ampoule, 10vials/pack |
3. Product Feature and Application
Bioendo CSE was labeled by the potency and matched to Lyophilized Amebocyte Lysate reagent lots. Users do not need to do the CSE/RSE ratio assay. Low potency control standard endotoxin is available to avoid lots of steps of dilution to provide convenience for end users.
Product Condition:
Control Standard Endotoxin (CSE), extracted from E.coli O111:B4, is an economic alternative to Reference Standard Endotoxin (RSE) in constructing standard curves, validating product and preparing controls in endotoxin test. The potency of CSE is referenced against USP Reference Standard Endotoxin, and labeled in the Certificate of Analysis.
Endotoxin test assay: Lysate reagent and CSE lot number have to be matched.
Pyrogen free tip box
Endotoxin free tubes
Product detail pictures:


Related Product Guide:
Our mission should be to turn out to be an innovative supplier of high-tech digital and communication devices by furnishing benefit added design and style, world-class manufacturing, and repair capabilities for 2022 China New Design LAL test for endotoxin - Control Standard Endotoxin (CSE) – Bioendo , The product will supply to all over the world, such as: South Korea, Milan, United Arab Emirates, We have a dedicated and aggressive sales team, and many branches, catering to our customers. We are looking for long-term business partnerships, and ensure our suppliers that they will definitely benefit in both short and long run.
Company director has very rich management experience and strict attitude, sales staff are warm and cheerful, technical staff are professional and responsible,so we have no worry about product,a nice manufacturer.