Top Suppliers Dry Heat Sterilizer Validation - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo
Top Suppliers Dry Heat Sterilizer Validation - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo Detail:
Single-Channel Mechanical Pipettes (Semi-sterile)
1. Product Information
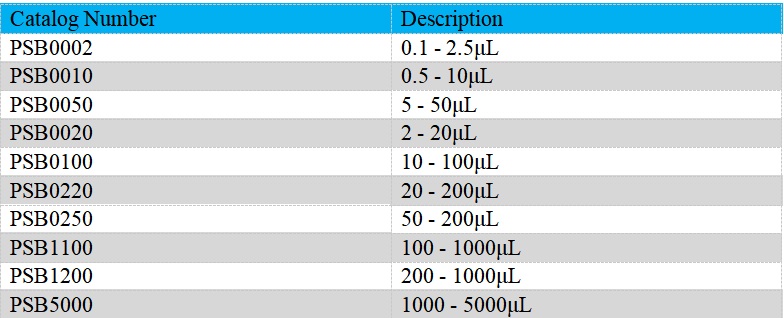
The Single-Channel Mechanical Pipettes (Semi-sterile) is adjustable, which could be used to dispense liquid precisely. Measuring volume of our Single-Channel Mechanical Pipettes (Semi-sterile) ranges from 0.1μL to 5mL. Products are produced based on ISO8655/DIN12650. It could be widely employed on endotoxin detection, etc..
2. Product Features
- Light weight, economic
- The pipettes cover volume range from 0.1μL to 5mL
- Semi-sterile
- Easy calibration and maintenance
Product detail pictures:

Related Product Guide:
Our enterprise insists all along the standard policy of "product high-quality is base of business survival; client satisfaction could be the staring point and ending of an business; persistent improvement is eternal pursuit of staff" as well as consistent purpose of "reputation first, client first" for Top Suppliers Dry Heat Sterilizer Validation - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo , The product will supply to all over the world, such as: Kyrgyzstan, Ghana, Nepal, We strongly believe that technology and service is our base today and quality will create our reliable walls of future. Only we have better and better quality , could we achieve our customers and ourselves, too. Welcome customers all over the word to contact us for getting further business and reliable relationships. We are always here working for your demands whenever you need.
The enterprise has a strong capital and competitive power, product is sufficient, reliable, so we have no worries on cooperating with them.








