Top Suppliers Dry Heat Sterilizer Validation - Mini Dry Heat Incubator – Bioendo
Top Suppliers Dry Heat Sterilizer Validation - Mini Dry Heat Incubator – Bioendo Detail:
Dry heat incubator single module
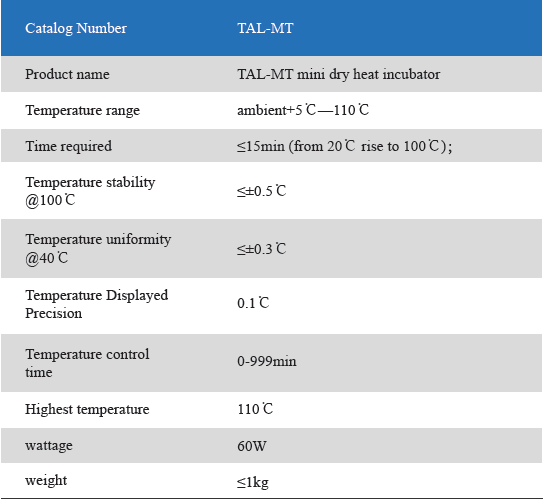
1. Product information
The Mini Dry Heat Incubator is a micro-processor controlled heating block with semi conductor heating technology.It adapts onboard use, smart, light and convenient for movement, suit for any kind of occasions. Especially good for the incubation of the gel clot LAL assay, LAL chromogenic endpoint assay incubation.
2. Product features
1. Unique designed. Smart and light, convenient movement, suit for various occasions.
2. LCD simultaneously display setting and actual time and temperature.Temperature calibration function.
3. Automatic fault detection function with buzzer alarm.
4. 24V DC input power, built-in over-temperature protection device.
5. Various of blocks for optional choice. Convenient for replacement. Easy cleaning and disinfection.
Product detail pictures:



Related Product Guide:
Our company has been concentrating on brand strategy. Customers' pleasure is our greatest advertising. We also source OEM service for Top Suppliers Dry Heat Sterilizer Validation - Mini Dry Heat Incubator – Bioendo , The product will supply to all over the world, such as: Australia, Eindhoven, Australia, Honest to every customers are our requested! First-class serve, best quality, best price and fastest delivery date is our advantage! Give every customers good serve is our tenet! This makes our company get the favour of customers and support! Welcome all over the world customers send us enquiry and looking forward your good co-operation !Please your inquiry for more details or request for dealership in selected regions.
Managers are visionary, they have the idea of "mutual benefits, continuous improvement and innovation", we have a pleasant conversation and Cooperation.








