Special Design for Bacterial Endotoxin Test Methods - Depyrogenated Sample Bottles ( Depyrogenated Galssware ) – Bioendo
Special Design for Bacterial Endotoxin Test Methods - Depyrogenated Sample Bottles ( Depyrogenated Galssware ) – Bioendo Detail:
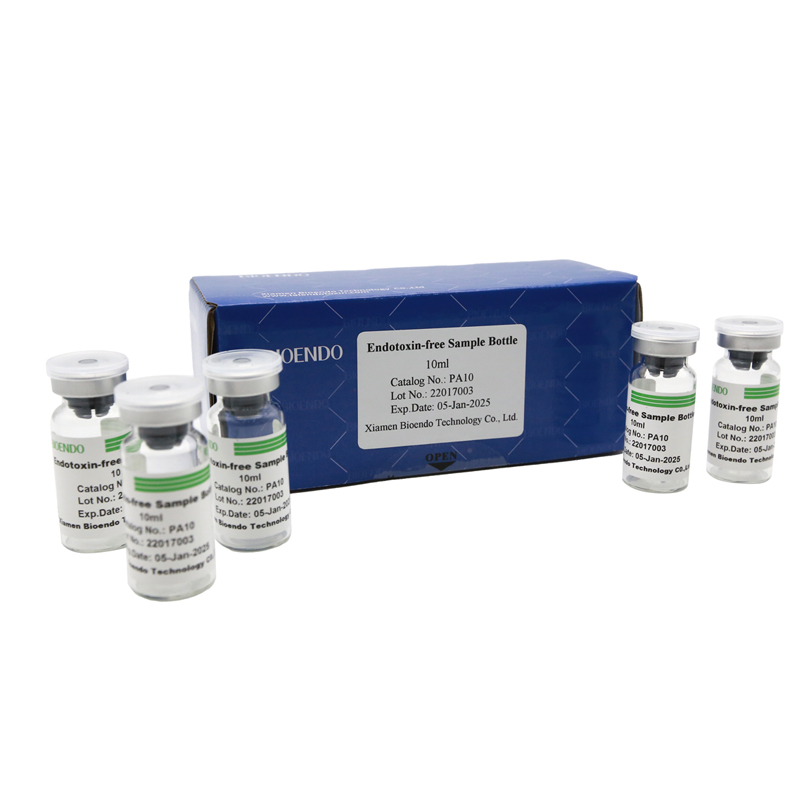
Depyrogenated Sample Bottle
1. Product information
We offer various of low endotoxin, pyrogen free accessories products,includes Water for Bacterial Endotoxins Test, pyrogen-free test tubes, pyrogen-free pipette tips, pyrogen free microplates and sample bottles for your conveniences. Amoung the sample bottle have two types, one is depyrogenated glassware and the other is depyrogenated plasticware, both endotoxin free level. High quality depyrogenated low endotoxin pyrogen free products insure the success of your experiments.
Depyrogenated (Endotoxin Free) Sample container(endotoxin free bottle, pyrogen free bottle, pyrogen free sample bottle)are glass bottles contain less than 0.005 EU/ml endotoxin. These bottles could be used to store various samples for lps endotoxin test, such as protein solution, vaccines, DNA solutions, dialysate, water for injections, etc., for endotoxin testing. Come with endotoxin free seals.
2. Product parameter
Endotoxin level < 0.005 EU/ml
3. Product features and application
For preparing and storage of the test samples.
| Catalog Number | Descriptions | Package |
| PA2 | Endotoxin free sample glass ampoule, 2ml | 10Pcs/Pack |
| PA10 | Endotoxin free sample glass vial, 10ml | 10Pcs/Pack or 110Pcs/Pack |
| PA50 | Endotoxin free sample glass vial, 50ml | 10Pcs/Pack |
| PA125 | Pyrogen free sample bottle, 125ml | 1Pcs/Pack |
| PA500 | Pyrogen free sample bottle, 500ml | 1Pcs/Pack |
Product detail pictures:





Related Product Guide:
We are also focusing on enhancing the things administration and QC program in order that we could keep fantastic advantage within the fiercely-competitive enterprise for Special Design for Bacterial Endotoxin Test Methods - Depyrogenated Sample Bottles ( Depyrogenated Galssware ) – Bioendo , The product will supply to all over the world, such as: Oman, Vancouver, Ghana, We adhere to client 1st, top quality 1st, continuous improvement, mutual advantage and win-win principles. When cooperation together with the customer, we provide shoppers with the highest high-quality of service. Established good business relations using the Zimbabwe buyer inside the business, we've got established own brand and reputation. At the identical time, wholeheartedly welcome new and old prospects to our company to go to and negotiate small business.
This is a honest and trustworthy company, technology and equipment are very advanced and the prodduct is very adequate, there is no worry in the suppliment.







