PriceList for LAL Method - Endotoxin Assay and (1,3)-ß-D-glucan assay software – Bioendo
PriceList for LAL Method - Endotoxin Assay and (1,3)-ß-D-glucan assay software – Bioendo Detail:
Endotoxin and (1,3)-ß-D-glucan assay software
1. Product information
Endotoxin and (1,3)-ß-D-glucan assay software is a powerful kinetic data analysis software,which gives the user data acquisition and processing of the maximum flexibility.
Features:
• Apply to Endotoxin assay,(1,3)-ß-D-glucan assay and ELISA data analysis
• With standard version and clinical diagnostic version for the different user groups.
• Data could be output transitions and connected to the LIS system.
• Customizable endotoxin test reports.
• Data analyzed by onset time, average rate, maximum rate and other methods.
• Data linear fitting or polynomial fitting.
• Real-time backup of the original data read.
• Integration of variety of kinetic microplate readers.
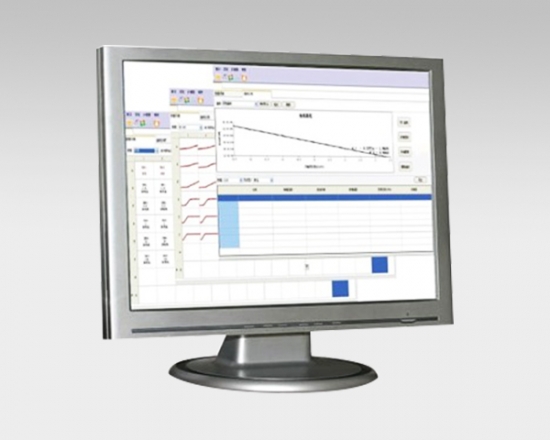
Product detail pictures:

Related Product Guide:
The pretty loaded projects management experiences and one to a person support model make the high importance of business enterprise communication and our easy understanding of your expectations for PriceList for LAL Method - Endotoxin Assay and (1,3)-ß-D-glucan assay software – Bioendo , The product will supply to all over the world, such as: United Arab Emirates, Mexico, French, High output volume, top quality, timely delivery and your satisfaction are guaranteed. We welcome all inquiries and comments. If you are interested in any of our products or have an OEM order to fulfill, please feel free to contact us now. Working with us will save you money and time.
The manufacturer gave us a big discount under the premise of ensuring the quality of products, thank you very much, we will select this company again.






