PriceList for Endotoxin Gel Clot Assay – Gel Clot Lyophilized Amebocyte Lysate Single Test in Vial – Bioendo
PriceList for Endotoxin Gel Clot Assay – Gel Clot Lyophilized Amebocyte Lysate Single Test in Vial – Bioendo Detail:
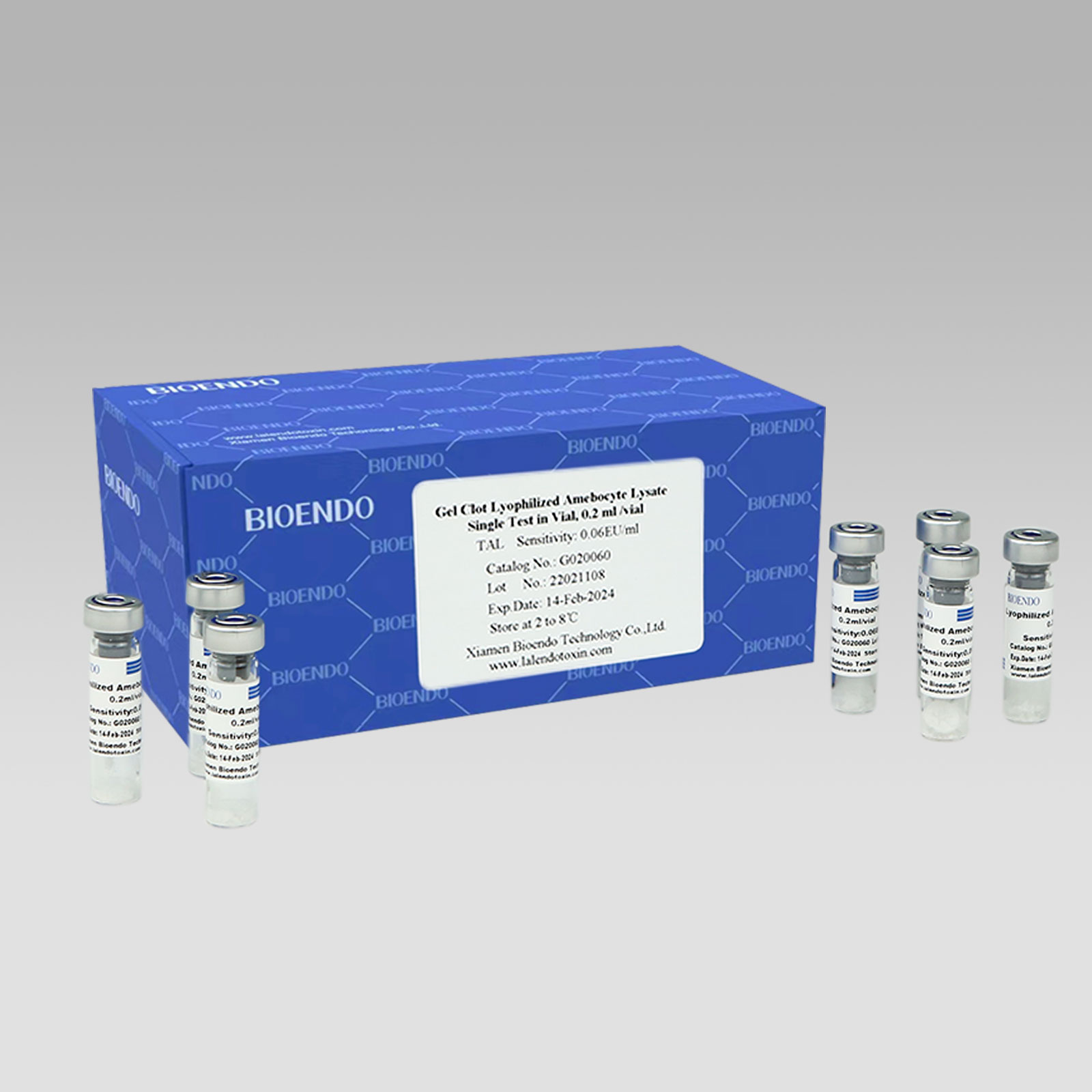
Gel Clot Lyophilized Amebocyte Lysate Single Test in Vial
1. Product Information
Gel Clot Lyophilized Amebocyte Lysate Single Test in Vial Contain endotoxin-specific Amebocyte Lysate which includes beta-glucan inhibitor in the formulation and will not react to beta-glucan. For our Single Test in Vial, you could add sample to the vial directly. This means you don’t need to reconstitute Amebocyte Lysate at first, and you will decide how many vials use each time to avoid waste. Endotoxin free tube for dilution of CSE is necessary. Operation of endotoxin detection by choice of Bioendo Gel Clot Lyophilized Amebocyte Lysate Single Test in Vial conforms to the USP, the EP.
2. Product Parameter
Gel clot assay single test glass vial
Sensitivities: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25 EU/ml
3.Product Feature and Application
Single step endotoxin detection,
without expensive endotoxin assay instruments,
Suitable for end-product endotoxin testing before product released,
Product sensitivity standardized according to US Pharmacopoeia criterion and China Pharmacopoeia criterion.
Note:
Lyophilized Amebocyte Lysate (LAL) reagent manufactured by Bioendo is made from lysate of amebocytes (white blood cells) from the horseshoe crab.
| Catalog number | Sensitivity (EU/ml) (IU/ml) | Tests/Vial | Vials/Pack |
| G020015 | 0.015 | 1 | 50 |
| G020030 | 0.03 | 1 | 50 |
| G020060 | 0.06 | 1 | 50 |
| G020125 | 0.125 | 1 | 50 |
| G020250 | 0.25 | 1 | 50 |
| G020500 | 0.5 | 1 | 50 |
Product Condition
The sensitivity of Lyophilized Amebocyte Lysate and potency of Control Standard Endotoxin are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come with product instruction, Certificate of Analysis.
Why the most selected gel clot assay kit G02:
1. The most economical and commonly used test reagent for endotoxin detection.
2. Single test in a vial for one stepping reduce the risk of contamination.
3. Gel clot assay single test glass vial no need sophisticated microplate reader.
4. Saving endotoxin free tube when using G02 series to test endotoxins in the procedure of endotoxin test assay’s operation.
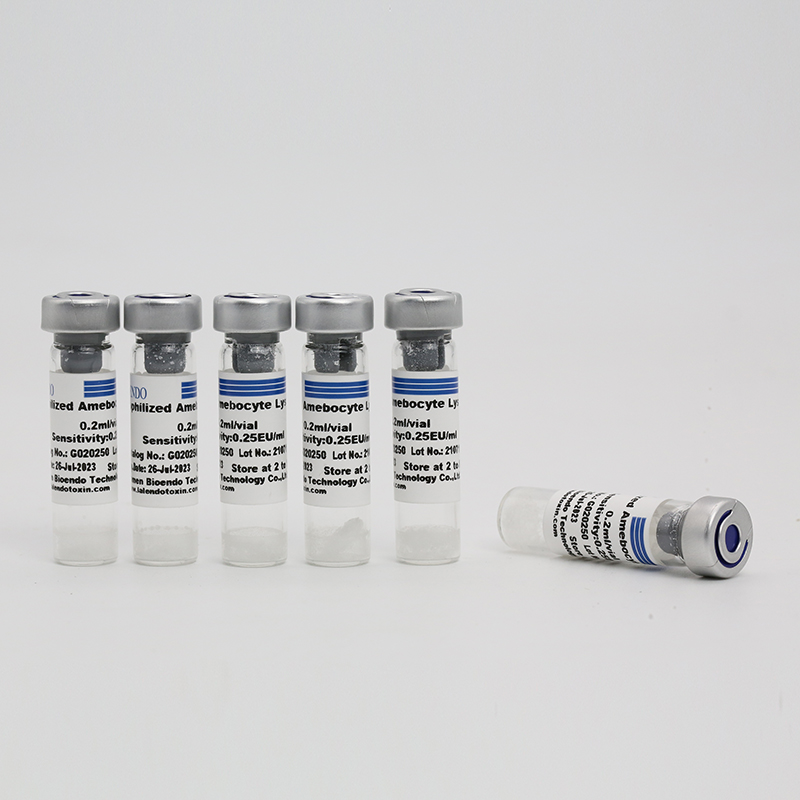
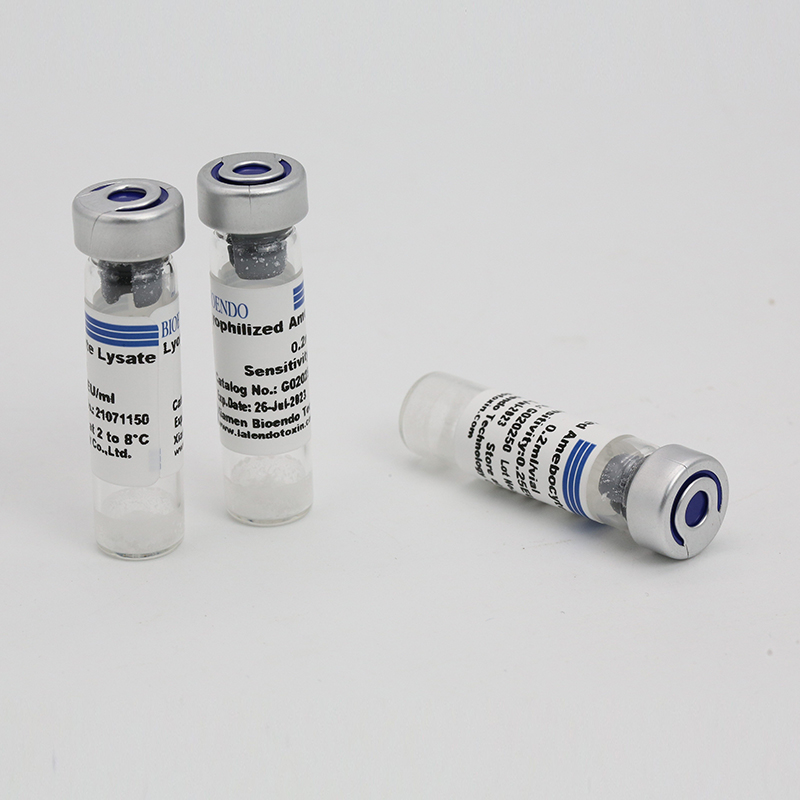
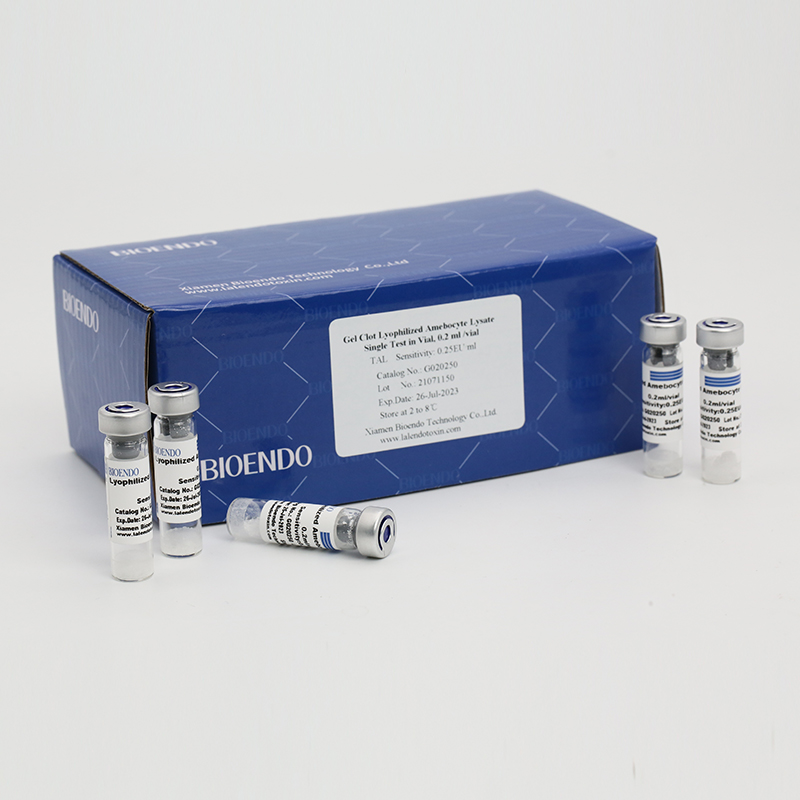
Product detail pictures:
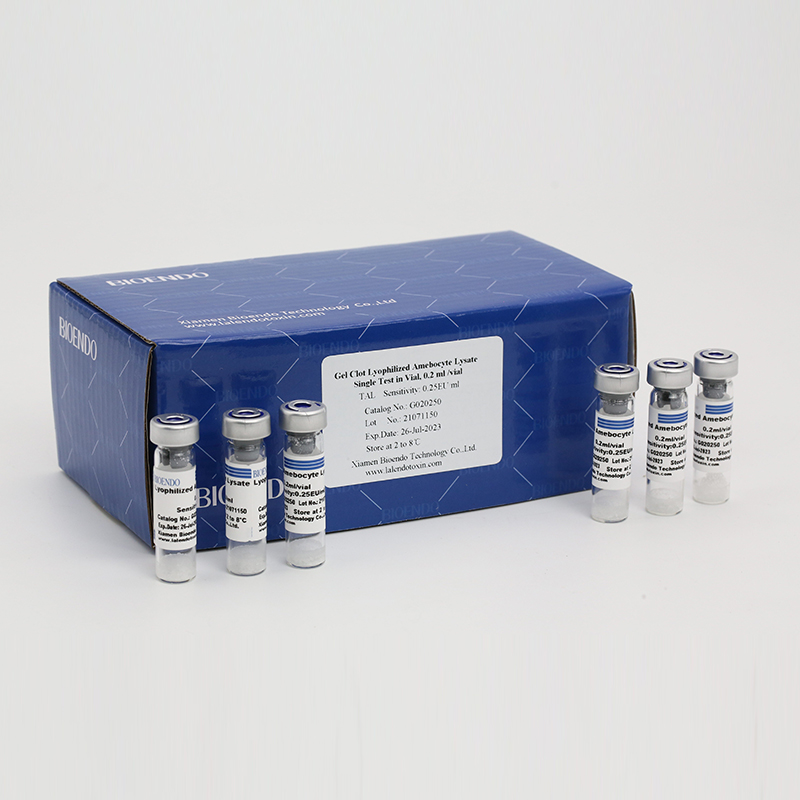
Related Product Guide:
Responsible excellent and fantastic credit rating standing are our principles, which will help us at a top-ranking position. Adhering towards the tenet of "quality initial, buyer supreme" for PriceList for Endotoxin Gel Clot Assay – Gel Clot Lyophilized Amebocyte Lysate Single Test in Vial – Bioendo , The product will supply to all over the world, such as: Cyprus, Albania, Istanbul, We are your reliable partner in international markets with the best quality products. Our advantages are innovation, flexibility and reliability which have been built during the last twenty years. We focus on providing service for our clients as a key element in strengthening our long-term relationships. Our continual availability of high grade products in combination with our excellent pre-sales and after-sales service ensures strong competitiveness in an increasingly globalized market.
The customer service reprersentative explained very detailed, service attitude is very good, reply is very timely and comprehensive, a happy communication! We hope to have a opportunity to cooperate.







