OEM/ODM Supplier Kit LAL - Endotoxin Assay and (1,3)-ß-D-glucan assay software – Bioendo
OEM/ODM Supplier Kit LAL - Endotoxin Assay and (1,3)-ß-D-glucan assay software – Bioendo Detail:
Endotoxin and (1,3)-ß-D-glucan assay software
1. Product information
Endotoxin and (1,3)-ß-D-glucan assay software is a powerful kinetic data analysis software,which gives the user data acquisition and processing of the maximum flexibility.
Features:
• Apply to Endotoxin assay,(1,3)-ß-D-glucan assay and ELISA data analysis
• With standard version and clinical diagnostic version for the different user groups.
• Data could be output transitions and connected to the LIS system.
• Customizable endotoxin test reports.
• Data analyzed by onset time, average rate, maximum rate and other methods.
• Data linear fitting or polynomial fitting.
• Real-time backup of the original data read.
• Integration of variety of kinetic microplate readers.
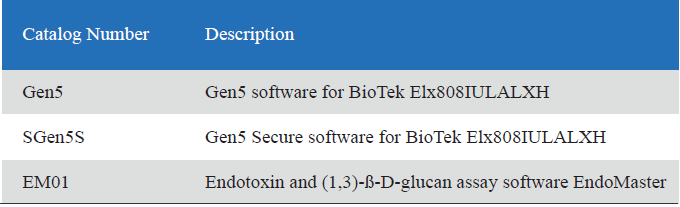
Product detail pictures:


Related Product Guide:
We now have a specialist, efficiency workforce to deliver excellent service for our purchaser. We always follow the tenet of customer-oriented, details-focused for OEM/ODM Supplier Kit LAL - Endotoxin Assay and (1,3)-ß-D-glucan assay software – Bioendo , The product will supply to all over the world, such as: Denver, Canada, Hungary, Our solutions are produced with the best raw materials. Every moment, we constantly improve the production programme. In order to ensure better quality and service, we now have been focusing on the production process. We have got high praise by partner. We are looking forward to establishing business relationship with you.
It is really lucky to meet such a good supplier, this is our most satisfied cooperation, I think we will work again!