OEM/ODM Factory Application Of LAL Test - Single-Channel Mechanical Pipettor – Bioendo
OEM/ODM Factory Application Of LAL Test - Single-Channel Mechanical Pipettor – Bioendo Detail:
Single-Channel Mechanical Pipettor
1. Product Information
Single channel mechanical pipette is ideal tool to support endotoxin detection with Lyophilized Amebocyte Lysate which covers gel-clot technique, kinetic turbidimetric technique, kinetic chromogenic technique, and end-point chromogenic technique. All pipettors are produced by following ISO8655 – 2:2002. The quality control involves gravimetric testing of each pipette with distilled water at 22℃.
2. Product Features:
- Light weight, economic, low force design
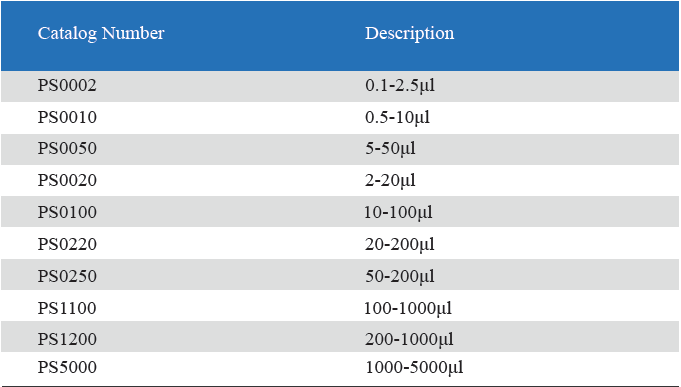
- Measuring volume range from 0.1μL to 5mL
- Easy to calibrate and maintain with tool supplied
- Design helps to avoid repetitive strain injuries
- Calibrated in accordance with ISO8655. Each pipettor supplied with individual test certificate
- The low part is available for autoclaving
Product detail pictures:

Related Product Guide:
Our enterprise since its inception, constantly regards product good quality as organization life, constantly improve production technology, strengthen merchandise high quality and continuously strengthen enterprise total good quality administration, in strict accordance with all the national standard ISO 9001:2000 for OEM/ODM Factory Application Of LAL Test - Single-Channel Mechanical Pipettor – Bioendo , The product will supply to all over the world, such as: Guatemala, Melbourne, Estonia, Our mission is "Provide Goods with Reliable Quality and Reasonable Prices". We welcome customers from every corner of the world to contact us for future business relationships and achieving mutual success!
High production efficiency and good product quality, fast delivery and completed after-sale protection, a right choice, a best choice.







