OEM Supply BET Test Method - Single-Channel Mechanical Pipettor – Bioendo
OEM Supply BET Test Method - Single-Channel Mechanical Pipettor – Bioendo Detail:
Single-Channel Mechanical Pipettor
1. Product Information
Single channel mechanical pipette is ideal tool to support endotoxin detection with Lyophilized Amebocyte Lysate which covers gel-clot technique, kinetic turbidimetric technique, kinetic chromogenic technique, and end-point chromogenic technique. All pipettors are produced by following ISO8655 – 2:2002. The quality control involves gravimetric testing of each pipette with distilled water at 22℃.
2. Product Features:
- Light weight, economic, low force design
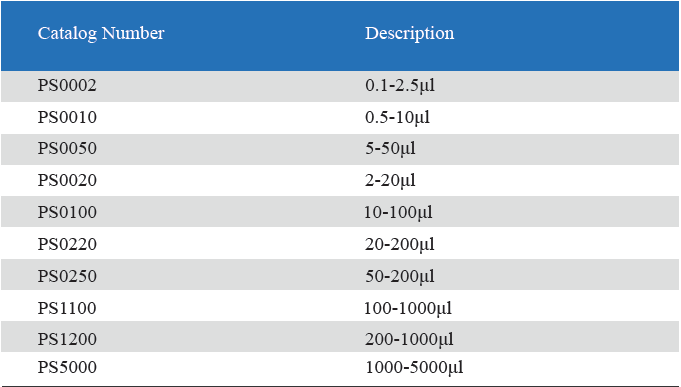
- Measuring volume range from 0.1μL to 5mL
- Easy to calibrate and maintain with tool supplied
- Design helps to avoid repetitive strain injuries
- Calibrated in accordance with ISO8655. Each pipettor supplied with individual test certificate
- The low part is available for autoclaving
Product detail pictures:

Related Product Guide:
Responsible excellent and fantastic credit rating standing are our principles, which will help us at a top-ranking position. Adhering towards the tenet of "quality initial, buyer supreme" for OEM Supply BET Test Method - Single-Channel Mechanical Pipettor – Bioendo , The product will supply to all over the world, such as: Angola, Bangalore, Singapore, Company name, is always regarding quality as company' s foundation, seeking for development via high degree of credibility , abiding by ISO quality management standard strictly, creating top-ranking company by spirit of progress-marking honesty and optimism.
The customer service reprersentative explained very detailed, service attitude is very good, reply is very timely and comprehensive, a happy communication! We hope to have a opportunity to cooperate.