OEM Supply BET Test Method - Single-Channel Mechanical Pipettor – Bioendo
OEM Supply BET Test Method - Single-Channel Mechanical Pipettor – Bioendo Detail:
Single-Channel Mechanical Pipettor
1. Product Information
Single channel mechanical pipette is ideal tool to support endotoxin detection with Lyophilized Amebocyte Lysate which covers gel-clot technique, kinetic turbidimetric technique, kinetic chromogenic technique, and end-point chromogenic technique. All pipettors are produced by following ISO8655 – 2:2002. The quality control involves gravimetric testing of each pipette with distilled water at 22℃.
2. Product Features:
- Light weight, economic, low force design
- Measuring volume range from 0.1μL to 5mL
- Easy to calibrate and maintain with tool supplied
- Design helps to avoid repetitive strain injuries
- Calibrated in accordance with ISO8655. Each pipettor supplied with individual test certificate
- The low part is available for autoclaving
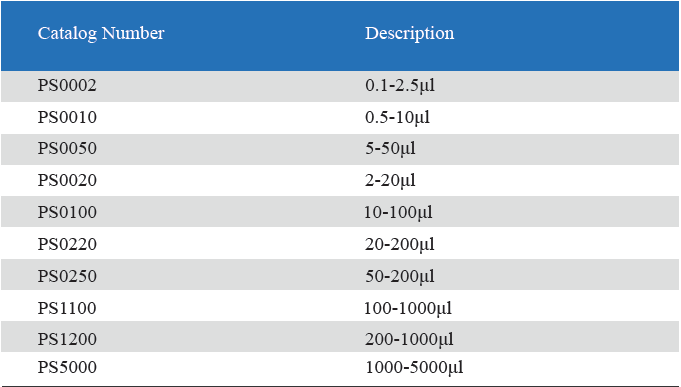
Product detail pictures:

Related Product Guide:
We have our own sales team, design team, technical team, QC team and package team. We have strict quality control procedures for each process. Also, all of our workers are experienced in printing field for OEM Supply BET Test Method - Single-Channel Mechanical Pipettor – Bioendo , The product will supply to all over the world, such as: Spain, Vietnam, Greenland, By adhering to the principle of "human oriented, winning by quality", our company sincerely welcomes merchants from at home and abroad to visit us, talk business with us and jointly create a brilliant future.
We have been appreciated the Chinese manufacturing, this time also did not let us disappoint,good job!