OEM Supply BET Test Method - Endotoxin Assay and (1,3)-ß-D-glucan assay software – Bioendo
OEM Supply BET Test Method - Endotoxin Assay and (1,3)-ß-D-glucan assay software – Bioendo Detail:
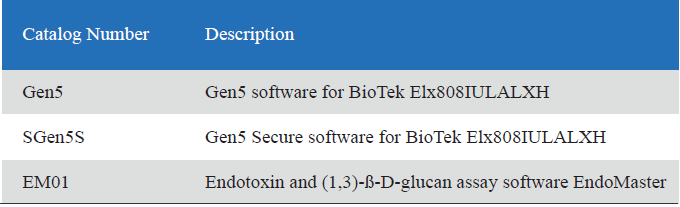
Endotoxin and (1,3)-ß-D-glucan assay software
1. Product information
Endotoxin and (1,3)-ß-D-glucan assay software is a powerful kinetic data analysis software,which gives the user data acquisition and processing of the maximum flexibility.
Features:
• Apply to Endotoxin assay,(1,3)-ß-D-glucan assay and ELISA data analysis
• With standard version and clinical diagnostic version for the different user groups.
• Data could be output transitions and connected to the LIS system.
• Customizable endotoxin test reports.
• Data analyzed by onset time, average rate, maximum rate and other methods.
• Data linear fitting or polynomial fitting.
• Real-time backup of the original data read.
• Integration of variety of kinetic microplate readers.
Product detail pictures:


Related Product Guide:
Fast and superior quotations, informed advisers to help you choose the correct merchandise that suits all your requirements, a short generation time, responsible quality control and different services for paying and shipping affairs for OEM Supply BET Test Method - Endotoxin Assay and (1,3)-ß-D-glucan assay software – Bioendo , The product will supply to all over the world, such as: Kuala Lumpur, Bahamas, Washington, We follow up the career and aspiration of our elder generation, and we are eager to open up a new prospect in this field, We insist on "Integrity, Profession, Win-win Cooperation", because we have a strong backup, that are excellent partners with advanced manufacturing lines, abundant technical strength, standard inspection system and good production capacity.
The enterprise has a strong capital and competitive power, product is sufficient, reliable, so we have no worries on cooperating with them.