OEM manufacturer BET Testing Method - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo
OEM manufacturer BET Testing Method - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo Detail:
Single-Channel Mechanical Pipettes (Semi-sterile)
1. Product Information
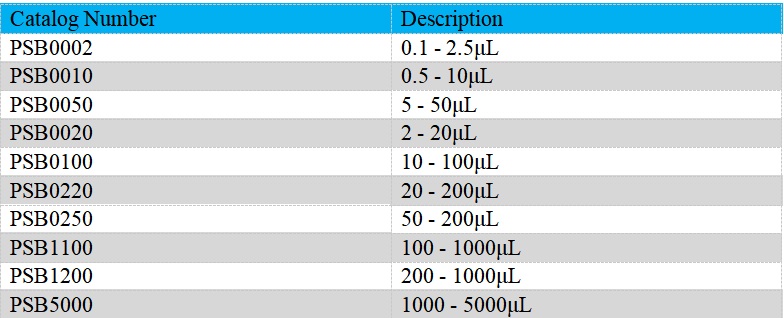
The Single-Channel Mechanical Pipettes (Semi-sterile) is adjustable, which could be used to dispense liquid precisely. Measuring volume of our Single-Channel Mechanical Pipettes (Semi-sterile) ranges from 0.1μL to 5mL. Products are produced based on ISO8655/DIN12650. It could be widely employed on endotoxin detection, etc..
2. Product Features
- Light weight, economic
- The pipettes cover volume range from 0.1μL to 5mL
- Semi-sterile
- Easy calibration and maintenance
Product detail pictures:

Related Product Guide:
We strive for excellence, services the customers", hopes to be the top cooperation team and dominator business for personnel, suppliers and prospects, realizes benefit share and continual promotion for OEM manufacturer BET Testing Method - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo , The product will supply to all over the world, such as: Jamaica, Leicester, Cancun, After years' creating and developing, with the advantages of trained qualified talents and rich marketing experience, outstanding achievements were gradually made. We get good reputation from the customers due to our good solutions quality and fine after-sale service. We sincerely wish to create a more prosperous and flourishing future together with all the friends home and abroad!
The company leader recept us warmly, through a meticulous and thorough discussion, we signed a purchase order. Hope to cooperate smoothly








