OEM manufacturer BET Testing Method - Mini Dry Heat Incubator – Bioendo
OEM manufacturer BET Testing Method - Mini Dry Heat Incubator – Bioendo Detail:
Dry heat incubator single module
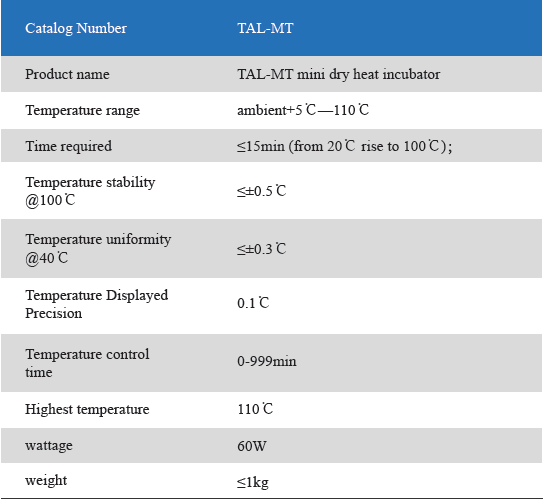
1. Product information
The Mini Dry Heat Incubator is a micro-processor controlled heating block with semi conductor heating technology.It adapts onboard use, smart, light and convenient for movement, suit for any kind of occasions. Especially good for the incubation of the gel clot LAL assay, LAL chromogenic endpoint assay incubation.
2. Product features
1. Unique designed. Smart and light, convenient movement, suit for various occasions.
2. LCD simultaneously display setting and actual time and temperature.Temperature calibration function.
3. Automatic fault detection function with buzzer alarm.
4. 24V DC input power, built-in over-temperature protection device.
5. Various of blocks for optional choice. Convenient for replacement. Easy cleaning and disinfection.
Product detail pictures:



Related Product Guide:
"Sincerity, Innovation, Rigorousness, and Efficiency" will be the persistent conception of our company to the long-term to establish together with customers for mutual reciprocity and mutual gain for OEM manufacturer BET Testing Method - Mini Dry Heat Incubator – Bioendo , The product will supply to all over the world, such as: Costa Rica, Norwegian, Juventus, Although continuous opportunity, we have now now developed serious a friendly relationship with many oversea merchants, such as ones through Virginia. We securely assume that the merchandise regarding t shirt printer machine is often good through a great number of having its good quality and also cost.
The sales person is professional and responsible, warm and polite, we had a pleasant conversation and no language barriers on communication.