OEM Manufacturer BET Test Procedure - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo
OEM Manufacturer BET Test Procedure - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo Detail:
Single-Channel Mechanical Pipettes (Semi-sterile)
1. Product Information
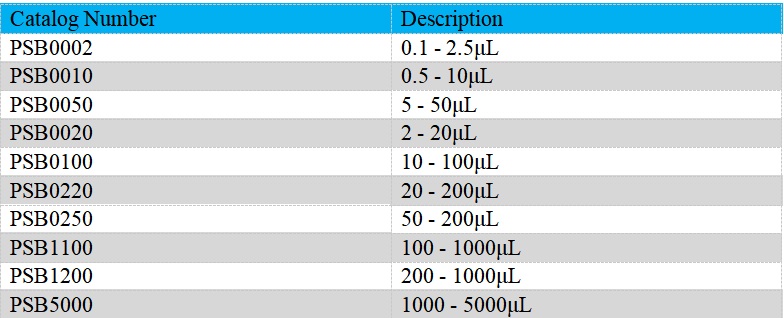
The Single-Channel Mechanical Pipettes (Semi-sterile) is adjustable, which could be used to dispense liquid precisely. Measuring volume of our Single-Channel Mechanical Pipettes (Semi-sterile) ranges from 0.1μL to 5mL. Products are produced based on ISO8655/DIN12650. It could be widely employed on endotoxin detection, etc..
2. Product Features
- Light weight, economic
- The pipettes cover volume range from 0.1μL to 5mL
- Semi-sterile
- Easy calibration and maintenance
Product detail pictures:

Related Product Guide:
Bear "Customer 1st, Good quality first" in mind, we work closely with our prospects and supply them with efficient and professional services for OEM Manufacturer BET Test Procedure - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo , The product will supply to all over the world, such as: Malta, Libya, Mecca, We will initiate the second phase of our development strategy. Our company regards "reasonable prices, efficient production time and good after-sales service" as our tenet. If you are interested in any of our products or would like to discuss a custom order, please feel free to contact us. We are looking forward to forming successful business relationships with new clients around the world in the near future.
This manufacturer can keep improving and perfecting products and service, it is in line with the rules of market competition, a competitive company.






