OEM Manufacturer BET Test Procedure - Rapid Gel Clot 10 Samples Kit – Bioendo
OEM Manufacturer BET Test Procedure - Rapid Gel Clot 10 Samples Kit – Bioendo Detail:
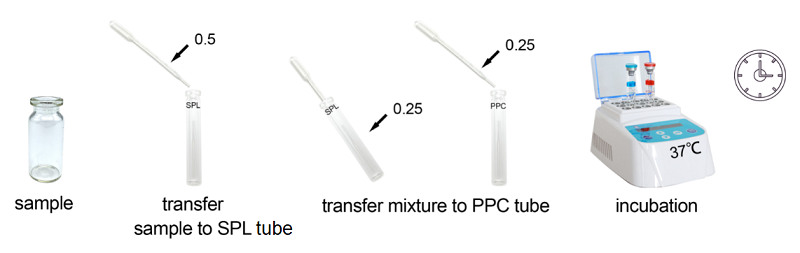
Bioendo Rapid Gel Clot Endotoxin Assay Kit is designed to rapidly quantify endotoxin in water or dialysate. Generally, RG kit’s result could be gained within 30 minutes. Under the guidance of detecting endotoxin in water or dialysate quickly, endotoxin detection with Bioendo Rapid Gel Clot Endotoxin Assay Kit does not need the multi steps’ dilution of Control Standard Endotoxin and test samples. Operation procedures are very convenient, additional experimental equipment are required. It is a convenient and rapid way to detect endotoxin in especial suitable for water or dialysate.
2. Product Parameter
Sensitivity Range: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25EU/ml, 0.5EU/ml
10 sample tests in the kit.
Assay time: less than 30 minutes
3. Product Application
Bioendo Rapid Gel Clot Endotoxin Assay Kit is designed to rapidly quantify endotoxin in water or dialysate as well as do quickly endotoxin detection in life science research.
Note:
Lyophilized Amebocyte Lysate (LAL) reagent manufactured by Bioendo is made from amebocyte lysate derived blood of horseshoe crab.
|
Catalog No. |
Description |
Kit Contents |
Sensitivity EU/ml |
Reaction Time minutes |
|
RG10025003 |
BioendoTM Rapid Gel Clot Endotoxin Assay Kit, 10 Samples Kit |
10 SPL Tubes; 10 PPC Tubes; 10 Endotoxin-free Sample Bottles; 10 Packs of (3pcs Transfer Pipettes) |
0.03 |
≤60 |
|
RG10025006 |
0.06 |
≤60 |
||
|
RG100250125 |
0.125 |
≤45 |
||
|
RG10025025 |
0.25 |
≤30 |
||
|
RG10025050 |
0.5 |
≤30 |
Product detail pictures:
Related Product Guide:
We believe that prolonged expression partnership is really a result of top of the range, value added support, rich encounter and personal contact for OEM Manufacturer BET Test Procedure - Rapid Gel Clot 10 Samples Kit – Bioendo , The product will supply to all over the world, such as: Russia, Czech Republic, Brasilia, Our products have enjoyed a great reputation for their good quality, competitive prices and prompt shipment in international market. Presently, we are sincerely looking forward to cooperating with more overseas customers based on mutual benefits.
The customer service reprersentative explained very detailed, service attitude is very good, reply is very timely and comprehensive, a happy communication! We hope to have a opportunity to cooperate.







