OEM Factory for BET Test Principle - Eight-channel Mechanical Pipette – Bioendo
OEM Factory for BET Test Principle - Eight-channel Mechanical Pipette – Bioendo Detail:
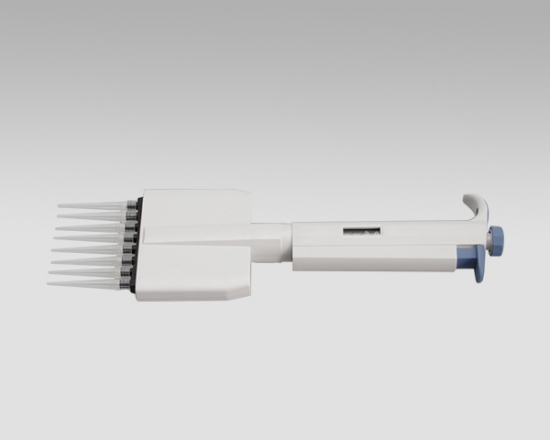
Eight-Channel Mechanical Pipettor
1. Product information
All multi-channel mechanical pipettor have been quality tested according to ISO8655-2:2002 with calibration certificate. The quality control involves gravimetric testing of each pipette with distilled water at 22℃. The multichannel mechanical pipettor is idea for the detectionof bacterial endotoxin lal endotoxin testing by kinetic turbidimetric andkinetic chromogenic method.
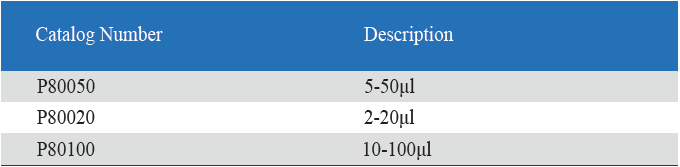
- Eight-Channel Mechanical Pipettor is available for standard 96-well plate
- Dispensing head rotates for optimum pipetting convenience
- Individual piston and tip cone assemblies allow easy repair and maintenance
- Compound material tip cone design allows visual seal verification
- Can be used with universal style pipette tips
-good for kinetic chromogenic, kinetic turbidimetric TAL orend-point chromogenic TAL endotoxin assay
Product detail pictures:
Related Product Guide:
We provide good power in high-quality and progress,merchandising,revenue and internet marketing and operation for OEM Factory for BET Test Principle - Eight-channel Mechanical Pipette – Bioendo , The product will supply to all over the world, such as: Costa Rica, Bangalore, Honduras, Item have passed by means of the national qualified certification and been well received in our main industry. Our expert engineering team will often be ready to serve you for consultation and feedback. We are able to also deliver you with cost-free samples to meet your specifications. Ideal efforts will probably be produced to provide you the most beneficial service and solutions. Should you be interested in our company and solutions, please make contact with us by sending us emails or call us straight away. To be able to know our solutions and enterprise. ar more, you'll be able to come to our factory to see it. We'll constantly welcome guests from all over the world to our firm. o build business enterprise. elations with us. Please feel absolutely free to speak to us for organization. nd we believe we will share the best trading practical experience with all our merchants.
The customer service staff's attitude is very sincere and the reply is timely and very detailed, this is very helpful for our deal,thank you.