OEM Customized Endotixins - Endotoxin Assay and (1,3)-ß-D-glucan assay software – Bioendo
OEM Customized Endotixins - Endotoxin Assay and (1,3)-ß-D-glucan assay software – Bioendo Detail:
Endotoxin and (1,3)-ß-D-glucan assay software
1. Product information
Endotoxin and (1,3)-ß-D-glucan assay software is a powerful kinetic data analysis software,which gives the user data acquisition and processing of the maximum flexibility.
Features:
• Apply to Endotoxin assay,(1,3)-ß-D-glucan assay and ELISA data analysis
• With standard version and clinical diagnostic version for the different user groups.
• Data could be output transitions and connected to the LIS system.
• Customizable endotoxin test reports.
• Data analyzed by onset time, average rate, maximum rate and other methods.
• Data linear fitting or polynomial fitting.
• Real-time backup of the original data read.
• Integration of variety of kinetic microplate readers.
Product detail pictures:

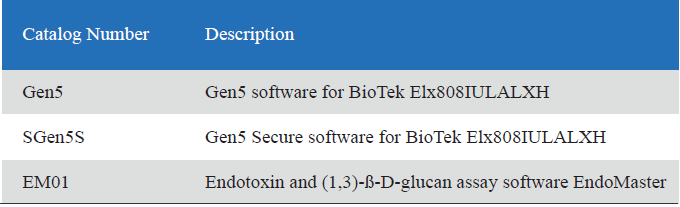
Related Product Guide:
Our mission will be to become an innovative supplier of high-tech digital and communication devices by furnishing benefit added structure, world-class manufacturing, and service capabilities for OEM Customized Endotixins - Endotoxin Assay and (1,3)-ß-D-glucan assay software – Bioendo , The product will supply to all over the world, such as: Slovak Republic, Florida, Seattle, We would very much welcome an opportunity to do business with you and have pleasure in attaching further details of our products. Excellent quality, competitive prices,punctual delivery and dependable service can be guaranteed.
In China, we have many partners, this company is the most satisfying to us, reliable quality and good credit, it is worth appreciation.