New Arrival China Gel Clot Endotoxin Test - Bioendo GC Endotoxin Test Kit (Gel Clot Assay) – Bioendo
New Arrival China Gel Clot Endotoxin Test - Bioendo GC Endotoxin Test Kit (Gel Clot Assay) – Bioendo Detail:
Bioendo GC Endotoxin Test Kit
Bioendo GC Endotoxin Test Kit (Gel Clot Assay)
1. Product Information
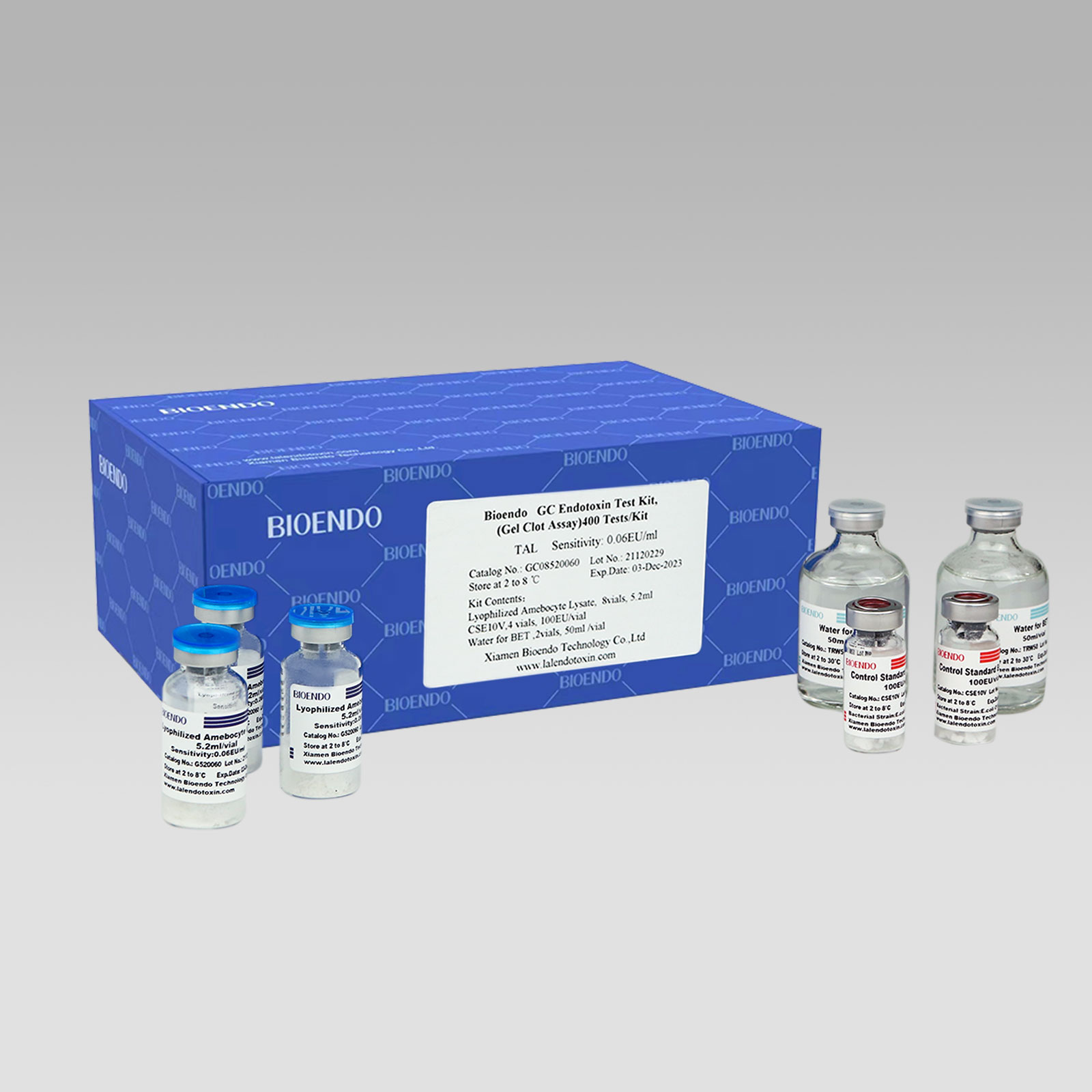
Bioendo GC Endotoxin Test Kit (Gel Clot Assay) contains 128 Tests/Kit, 400Tests/Kit, 1600Tests/Kit, and 4500 Tests/Kit. And sensitivity of Amebocyte Lysate included by the Kit is 0.03 EU/ml, 0.06 EU/ml, 0.125 EU/ml, 0.25 EU/ml, 0.5 EU/ml. The Kit contains Amebocyte Lysate, CSE and Water for BET. Four kinds of configuration of gel-clot endotoxin assay kit to satisfied the demand. This means you could choose different configuration kits according to the requirement.
2. Product Parameter
Sensitivities: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25EU/ml, 0.5 EU/ml
3.Product Feature and Application
Full range of sensitivities to meet the demand. No special instrument is needed for endotoxin detection with Bioendo GC Endotoxin Test Kit (Gel Clot Assay). It is the best choice when you need to do lots of endotoxin detection.
Note:
Lyophilized Amebocyte Lysate (LAL/TAL) reagent manufactured by Bioendo is made from amebocyte lysate derived blood of horseshoe crab.
|
Catalog No. |
Sensitivity (EU/ml) |
Description |
Kit Contents |
|
GC90520030 |
0.03 |
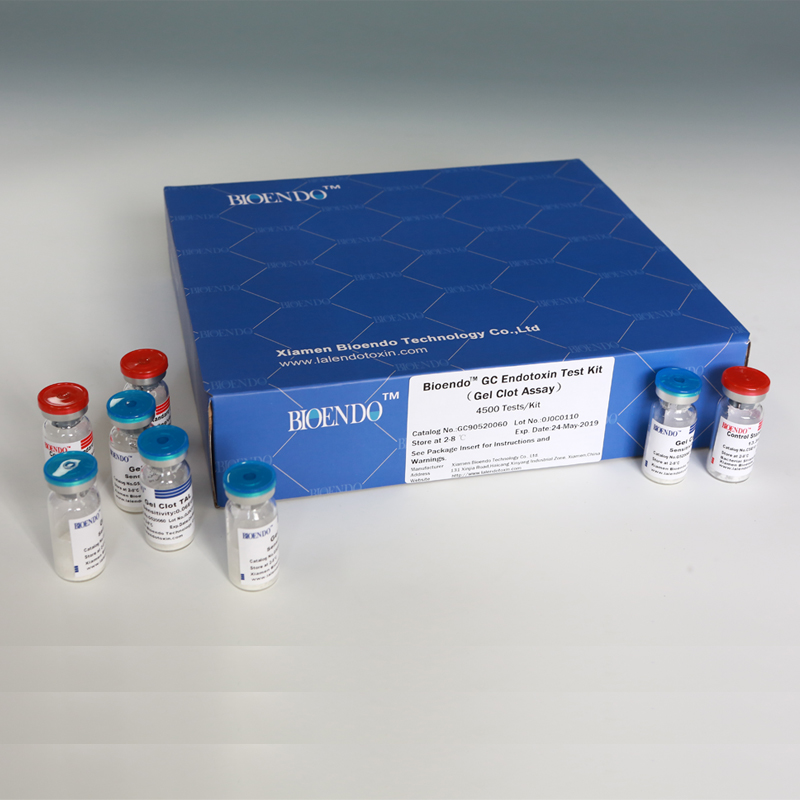
Bioendo™ GC Endotoxin Test Kit (Gel Clot Assay) 4500 Tests/Kit |
90 Gel Clot Lyophilized Amebocyte Lysate, 5.2ml (50 Tests/Vial); 20 CSE10V; |
|
GC90520060 |
0.06 |
||
|
GC90520125 |
0.125 |
||
|
GC90520250 |
0.25 |
||
|
GC90520500 |
0.5 |
||
|
GC08520030 |
0.03 |
Bioendo™ GC Endotoxin Test Kit (Gel Clot Assay) 400 Tests/Kit |
8 Gel Clot Lyophilized Amebocyte Lysate, 5.2ml (50 Tests/Vial); 4 CSE10V; 2 Water for BET, 50ml/vial; |
|
GC08520060 |
0.06 |
||
|
GC08520125 |
0.125 |
||
|
GC08520250 |
0.25 |
||
|
GC08520500 |
0.5 |
||
|
GC100170030 |
0.03 |
Bioendo™ GC Endotoxin Test Kit (Gel Clot Assay) 1600 Tests/Kit |
100 Gel Clot Lyophilized Amebocyte Lysate, 1.7ml (16 Tests/Vial); 10 CSE10V; |
|
GC100170060 |
0.06 |
||
|
GC100170125 |
0.125 |
||
|
GC100170250 |
0.25 |
||
|
GC100170500 |
0.5 |
||
|
GC08170030 |
0.03 |
Bioendo™ GC Endotoxin Test Kit (Gel Clot Assay) 128 Tests/Kit |
8 Gel Clot Lyophilized Amebocyte Lysate, 1.7ml (16 Tests/Vial); 4 CSE10V; 2 Water for BET, 50ml/vial; |
|
GC08170060 |
0.06 |
||
|
GC08170125 |
0.125 |
||
|
GC08170250 |
0.25 |
||
|
GC08170500 |
0.5 |
Product condition:
Endotoxin Test kit’s sensitivity and the Control Standard Endotoxin potency are assayed against USP Reference Standard Endotoxin. The Endotoxin Test reagentkits come with product instruction, Certificate of Analysis.
Product detail pictures:

Related Product Guide:
Well-run gear, qualified revenue workforce, and superior after-sales companies; We've been also a unified huge loved ones, anyone persist with the organization benefit "unification, determination, tolerance" for New Arrival China Gel Clot Endotoxin Test - Bioendo GC Endotoxin Test Kit (Gel Clot Assay) – Bioendo , The product will supply to all over the world, such as: Porto, Belgium, Bulgaria, They're durable modeling and promoting effectively all over the world. Under no circumstances disappearing major functions in a quick time, it's a should for you of excellent good quality. Guided by the principle of "Prudence, Efficiency, Union and Innovation. the company make a terrific efforts to expand its international trade, raise its company profit and raise its export scale. We're confident that we are going to possess a vibrant prospect and to be distributed all over the world within the years to come.
Adhering to the business principle of mutual benefits, we have a happy and successful transaction, we think we will be the best business partner.








