Manufacturing Companies for Advantages Of LAL Test - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo
Manufacturing Companies for Advantages Of LAL Test - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo Detail:
Single-Channel Mechanical Pipettes (Semi-sterile)
1. Product Information
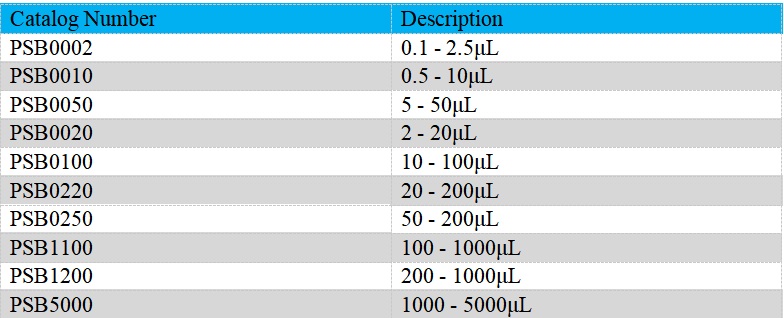
The Single-Channel Mechanical Pipettes (Semi-sterile) is adjustable, which could be used to dispense liquid precisely. Measuring volume of our Single-Channel Mechanical Pipettes (Semi-sterile) ranges from 0.1μL to 5mL. Products are produced based on ISO8655/DIN12650. It could be widely employed on endotoxin detection, etc..
2. Product Features
- Light weight, economic
- The pipettes cover volume range from 0.1μL to 5mL
- Semi-sterile
- Easy calibration and maintenance
Product detail pictures:

Related Product Guide:
We have now a skilled, performance group to offer excellent support for our consumer. We usually follow the tenet of customer-oriented, details-focused for Manufacturing Companies for Advantages Of LAL Test - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo , The product will supply to all over the world, such as: Manchester, Latvia, New Delhi, Our company has built stable business relationships with many well-known domestic companies as well as oversea customers. With the goal of providing high quality products to customers at low cots, we've been committed to improving its capacities in research, development, manufacturing and management. We have honored to receive recognition from our customers. Till now we have now passed ISO9001 in 2005 and ISO/TS16949 in 2008. Enterprises of "quality of survival, the credibility of development" for the purpose, sincerely welcome domestic and foreign businessmen to visit to discuss cooperation.
We have been engaged in this industry for many years, we appreciate the work attitude and production capacity of the company, this is a reputable and professional manufacturer.