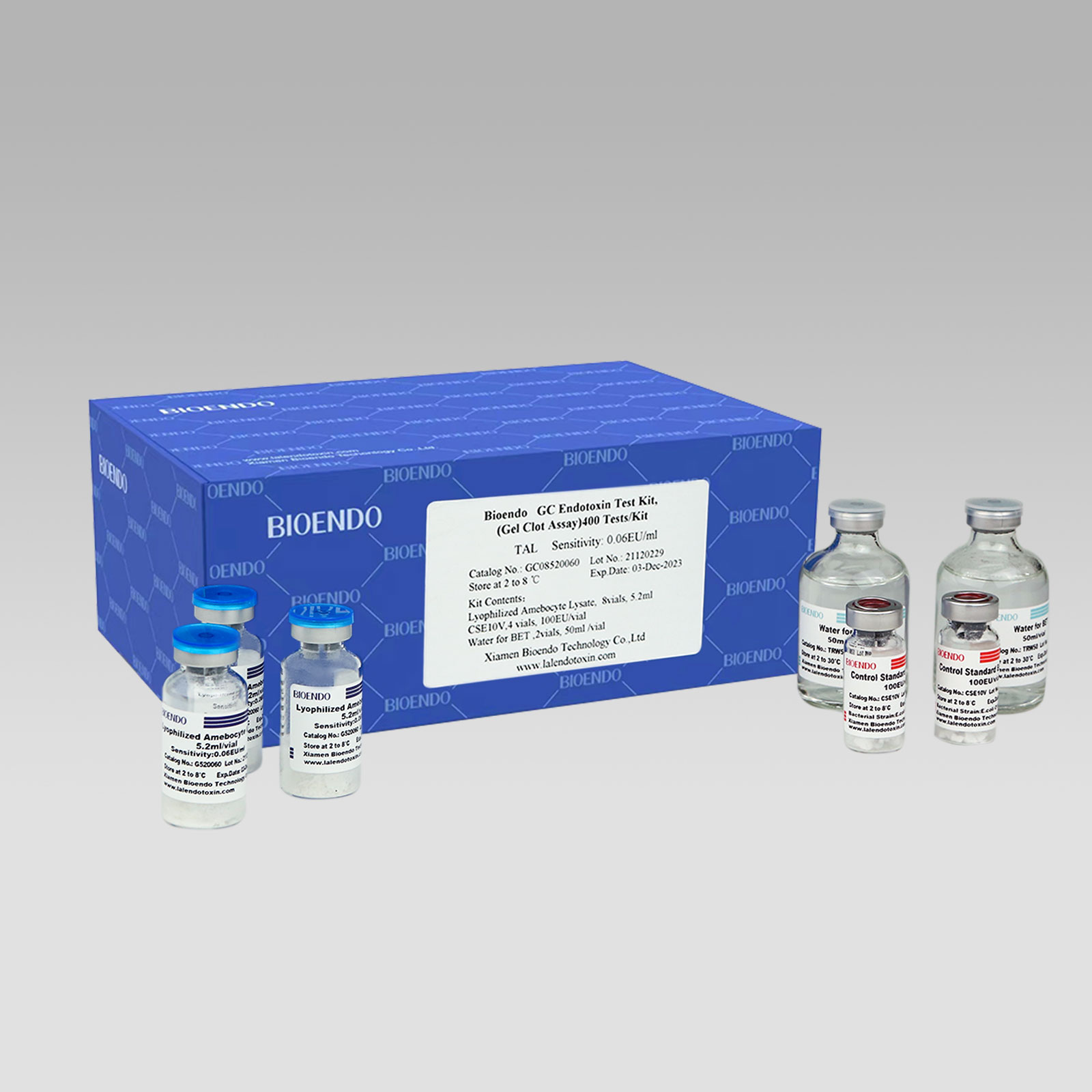
Hot New Products Rapid Gel Clot Endotoxin Test Kit For Water And Dialysate - Gel Clot Lyophilized Amebocyte Lysate Multi-test Vial G52 – Bioendo
Hot New Products Rapid Gel Clot Endotoxin Test Kit For Water And Dialysate - Gel Clot Lyophilized Amebocyte Lysate Multi-test Vial G52 – Bioendo Detail:
Gel Clot Method Lyophilized Amebocyte Lysate Multi-test Vial G52 series
1. Product Information
Gel Clot method Lyophilized Amebocyte Lysate Multi-test Vial is the Lyophilized Amebocyte Lysate reagent which select and use gel clot technique to detect endotoxin or pyrogen.
As the widespread method, gel-clot test for endotoxin is simple and does not require specific and expensive instrument. Bioendo provides Gel Clot Lyophilized Amebocyte Lysate reagent in 5.2ml per vial.
2. Product Parameters
Sensitivity range: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25EU/ml, 0.5 EU/ml
3. Product Application
End-product endotoxin (pyrogen) qualification, waterfor injection endotoxin assay, raw material endotoxin testing or endotoxinlevel monitoring during manufacturing process for pharmaceutical companies ormedical devices manufacturers.
Note:
Lyophilized Amebocyte Lysate (LAL) reagent manufactured by Bioendo is made from lysate of amebocytes (white blood cells) from the horseshoe crab.
| Catalog Number | Sensitivity (EU/ml or IU/ml) | ml/vial | Tests/Vial | Vials/Pack |
| G520030 | 0.03 | 5.2 | 50 | 10 |
| G520060 | 0.06 | 5.2 | 50 | 10 |
| G520125 | 0.125 | 5.2 | 50 | 10 |
| G520250 | 0.25 | 5.2 | 50 | 10 |
| G520500 | 0.5 | 5.2 | 50 | 10 |
Product condition:
The Lyophilized Amebocyte Lysate reagent sensitivity and the Control Standard Endotoxin potency are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte reagent kits come with product instruction, Certificate of Analysis, MSDS.
What is the difference between single test and multiple test?
● Single test: reconstitute the single test lysate reagent by BET water in the glass vial or glass ampoule.
● Multi-test: reconstitute the lysate reagent with BET water, and then add marked amount of lysate reagent following COA to the reaction tube or well plate for use. There is no difference in the sample pre-processing procedure; according to the amount of testing used, the sample size used for a single test is larger than the sample size used for multiple tests.
Why the most selected gel clot assay kit G52:
1. Multi test reagent for endotoxin detection in the applications of certain samples’ endotoxin detection.
2. G52 series of Gel clot assay multi test glass vial no need sophisticated microplate reader. Procedure of incubation by water bath or dry heat incubator.
3. High end quality of endotoxin free tube (<0.005EU/ml) and High quality of pyrogen free tips (<0.005EU/ml) as the guaranteed consumables to ensure the correct result.
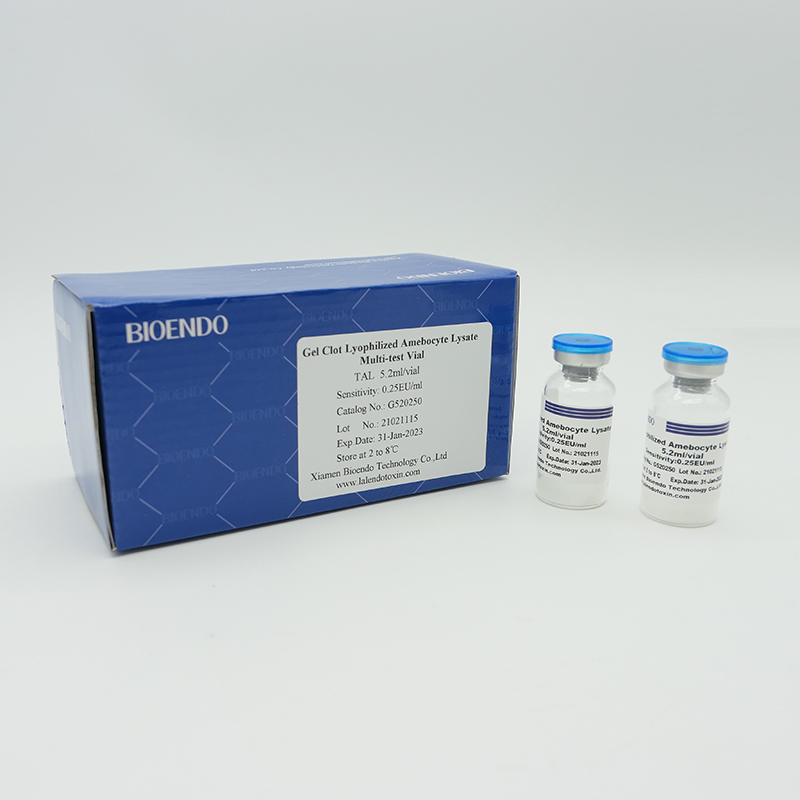
Product detail pictures:



Related Product Guide:
We now have several exceptional workers customers good at marketing, QC, and working with types of troublesome trouble during the creation system for Hot New Products Rapid Gel Clot Endotoxin Test Kit For Water And Dialysate - Gel Clot Lyophilized Amebocyte Lysate Multi-test Vial G52 – Bioendo , The product will supply to all over the world, such as: Dubai, Thailand, New Zealand, Whether selecting a current product from our catalog or seeking engineering assistance for your application, you can talk to our customer service center about your sourcing requirements. We can provided good quality with competitive price for you personally.
This is a very professional and honest Chinese supplier, from now on we fell in love with the Chinese manufacturing.