High definition Kinetic chromogenic LAL Assay - Bioendo KC Endotoxin Test Kit (Kinetic Chromogenic Assay) – Bioendo
High definition Kinetic chromogenic LAL Assay - Bioendo KC Endotoxin Test Kit (Kinetic Chromogenic Assay) – Bioendo Detail:
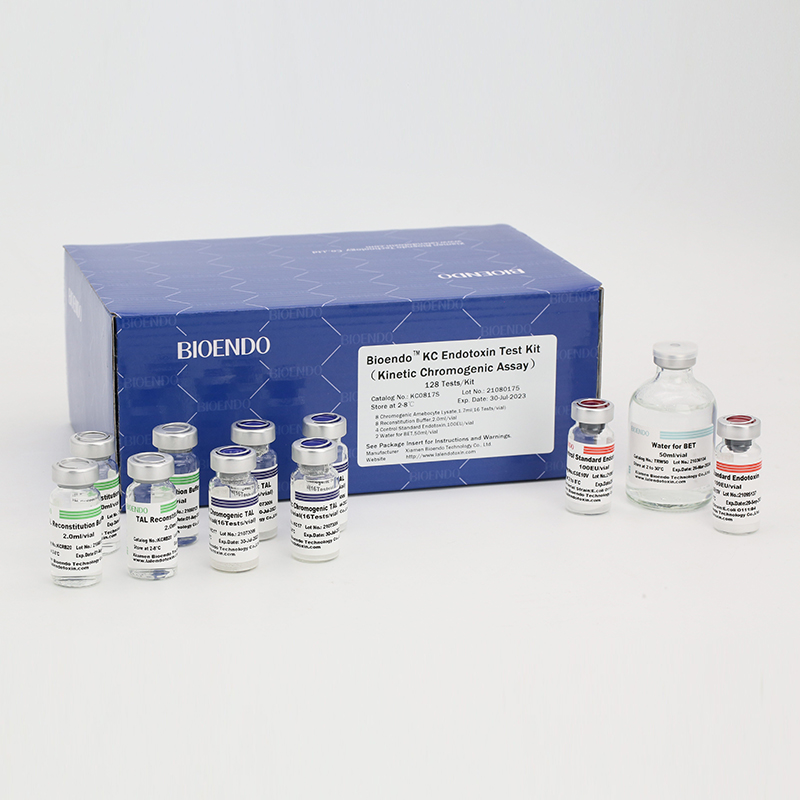
Bioendo KC Endotoxin Test Kit (Kinetic Chromogenic Assay)
1. Product Information
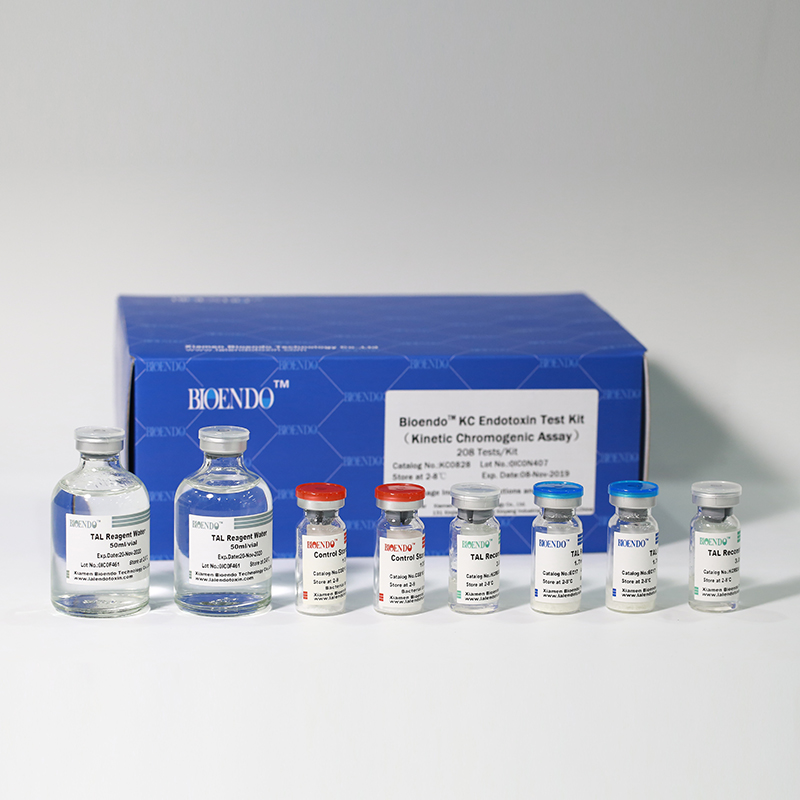
In Bioendo KC Endotoxin Test Kit, Amebocyte Lysate is co-lyophilized with chromogenic substrate. Therefore, bacterial endotoxin could be quantified based on the chromogenic reaction. The assay is strong resistance to interference, and has advantages of kinetic turbidimetric and end-point chromogenic method. Bioendo Endotoxin Test Kit contains Chromogenic Amebocyte Lysate, Reconstitution Buffer, CSE, Water for BET. Endotoxin detection with Kinetic Chromogenic method requires a kinetic incubating microplate reader such as ELx808IULALXH.
2. Product Parameter
Assay Range: 0.005 – 50EU/ml; 0.001 – 10EU/ml
|
Catalog No. |
Description |
Kit Contents |
Sensitivity EU/ml |
|
KC5028 |
Bioendo™ KC Endotoxin Test Kit (Kinetic Chromogenic Assay), 1300 Tests/Kit |
50 Chromogenic Amebocyte Lysate, 2.8ml (26 Tests/Vial); 50 Reconstitution Buffer, 3.0ml/vial; 10CSE; |
0.005-5EU/ml |
|
KC5028S |
0.001-10EU/ml |
||
|
KC0828 |
Bioendo™ KC Endotoxin Test Kit (Kinetic Chromogenic Assay), 208 Tests/Kit |
8 Chromogenic Amebocyte Lysate, 2.8ml (26 Tests/Vial); 8 Reconstitution Buffer, 3.0ml/vial; 4 CSE; 2 Water for BET, 50ml/vial; |
0.005-5EU/ml |
|
KC0828S |
0.001-10EU/ml |
||
|
KC5017 |
Bioendo™ KC Endotoxin Test Kit (Kinetic Chromogenic Assay), 800 Tests/Kit |
50 Chromogenic Amebocyte Lysate, 1.7ml (16 Tests/Vial); 50 Reconstitution Buffer, 2.0ml/vial; 10CSE; |
0.005-5 EU/ml |
|
KC5017S |
0.001-10 EU/m |
||
|
KC0817 |
Bioendo™ KC Endotoxin Test Kit (Kinetic Chromogenic Assay), 128 Tests/kit |
8 Kinetic Chromogenic Amebocyte Lysate, 1.7ml (16 Tests/vial); 8 Reconstitution Buffer, 2.0ml/vial; 4 CSE; 2 Water for BET, 50ml/vial; |
0.005-5 EU/ml |
|
KC0817S |
0.001-10 EU/ml |
3. Product Feature and Application
BioendoTM KC Endotoxin Test Kit (Kinetic Chromogenic Assay) features strong resistance to interference, and has advantages of kinetic turbidimetric and end-point chromogenic method. It is especially suitable for endotoxin detection of biological samples like vaccine, antibody, protein, nucleic acid, etc.
Note:
Lyophilized Amebocyte Lysate reagent manufactured by Bioendo is made from amebocyte lysate from the horseshoe crab (Tachypleus tridentatus).
Product Condition:
The sensitivity of Lyophilized Amebocyte Lysate and potency of Control Standard Endotoxin are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come with product instruction, Certificate of Analysis.
The kinetic chromogenic endotoxin test kit have to choose the microplate reader with 405nm filters.
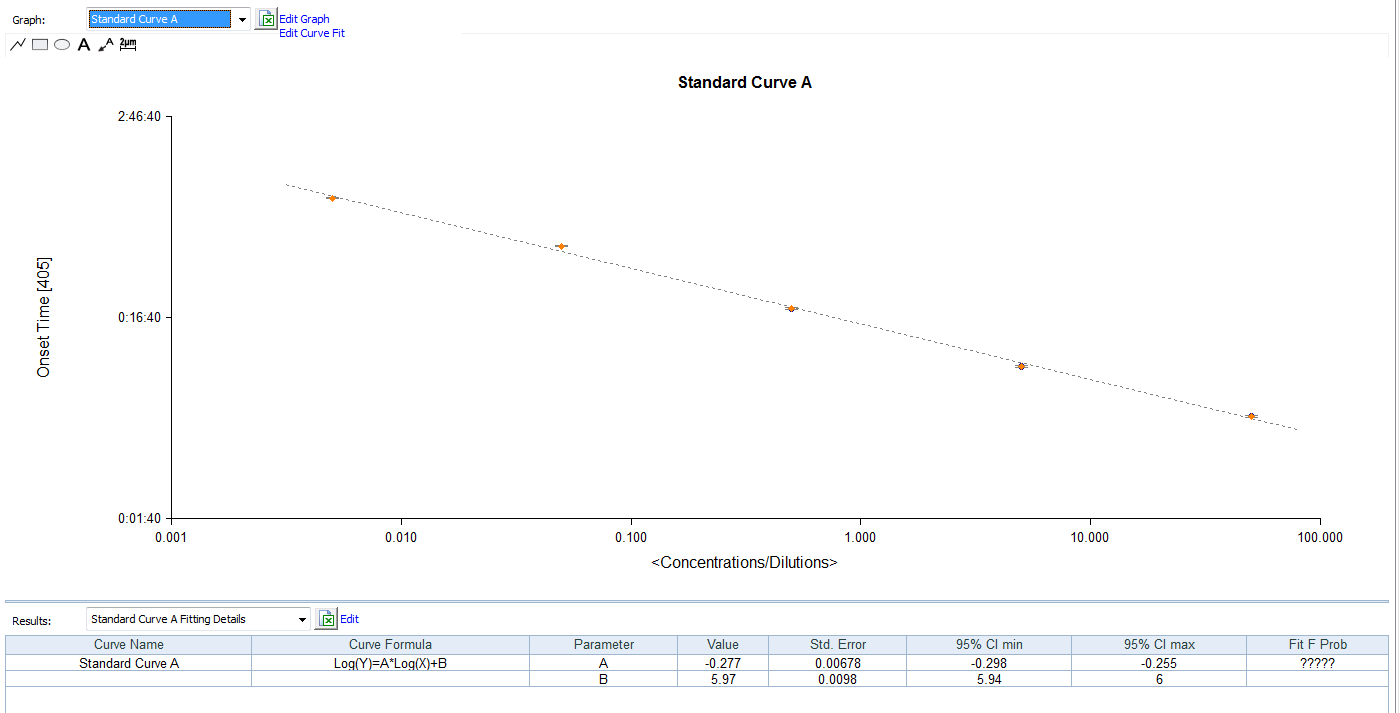
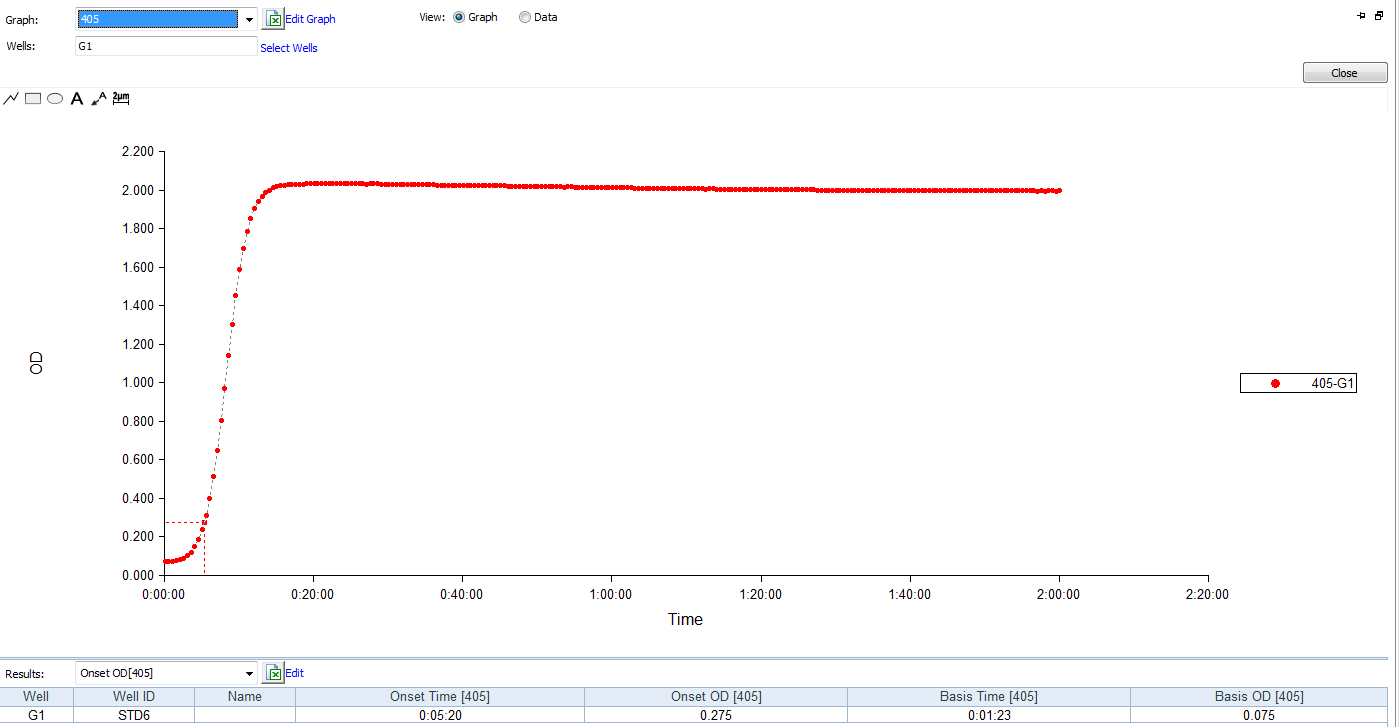
Product detail pictures:



Related Product Guide:
We also supply merchandise sourcing and flight consolidation companies. We now have our very own manufacturing facility and sourcing business. We could present you with almost every kind of product relevant to our solution array for High definition Kinetic chromogenic LAL Assay - Bioendo KC Endotoxin Test Kit (Kinetic Chromogenic Assay) – Bioendo , The product will supply to all over the world, such as: Montreal, Toronto, Nepal, They are sturdy modeling and promoting effectively all over the world. Never ever disappearing major functions within a quick time, it's a have to for you of fantastic good quality. Guided by the principle of "Prudence, Efficiency, Union and Innovation. the corporation. ake an excellent efforts to expand its international trade, raise its organization. rofit and raise its export scale. We are confident that we are going to have a bright prospect and to be distributed all over the world in the years to come.
The accounts manager made a detailed introduction about the product, so that we have a comprehensive understanding of the product, and ultimately we decided to cooperate.