Good quality Micro kinetic chromogenic endotoxin test kit - Bioendo KC Endotoxin Test Kit (Kinetic Chromogenic Assay) – Bioendo
Good quality Micro kinetic chromogenic endotoxin test kit - Bioendo KC Endotoxin Test Kit (Kinetic Chromogenic Assay) – Bioendo Detail:
Bioendo KC Endotoxin Test Kit (Kinetic Chromogenic Assay)
1. Product Information
In Bioendo KC Endotoxin Test Kit, Amebocyte Lysate is co-lyophilized with chromogenic substrate. Therefore, bacterial endotoxin could be quantified based on the chromogenic reaction. The assay is strong resistance to interference, and has advantages of kinetic turbidimetric and end-point chromogenic method. Bioendo Endotoxin Test Kit contains Chromogenic Amebocyte Lysate, Reconstitution Buffer, CSE, Water for BET. Endotoxin detection with Kinetic Chromogenic method requires a kinetic incubating microplate reader such as ELx808IULALXH.
2. Product Parameter
Assay Range: 0.005 – 50EU/ml; 0.001 – 10EU/ml
|
Catalog No. |
Description |
Kit Contents |
Sensitivity EU/ml |
|
KC5028 |
Bioendo™ KC Endotoxin Test Kit (Kinetic Chromogenic Assay), 1300 Tests/Kit |
50 Chromogenic Amebocyte Lysate, 2.8ml (26 Tests/Vial); 50 Reconstitution Buffer, 3.0ml/vial; 10CSE; |
0.005-5EU/ml |
|
KC5028S |
0.001-10EU/ml |
||
|
KC0828 |
Bioendo™ KC Endotoxin Test Kit (Kinetic Chromogenic Assay), 208 Tests/Kit |
8 Chromogenic Amebocyte Lysate, 2.8ml (26 Tests/Vial); 8 Reconstitution Buffer, 3.0ml/vial; 4 CSE; 2 Water for BET, 50ml/vial; |
0.005-5EU/ml |
|
KC0828S |
0.001-10EU/ml |
||
|
KC5017 |
Bioendo™ KC Endotoxin Test Kit (Kinetic Chromogenic Assay), 800 Tests/Kit |
50 Chromogenic Amebocyte Lysate, 1.7ml (16 Tests/Vial); 50 Reconstitution Buffer, 2.0ml/vial; 10CSE; |
0.005-5 EU/ml |
|
KC5017S |
0.001-10 EU/m |
||
|
KC0817 |
Bioendo™ KC Endotoxin Test Kit (Kinetic Chromogenic Assay), 128 Tests/kit |
8 Kinetic Chromogenic Amebocyte Lysate, 1.7ml (16 Tests/vial); 8 Reconstitution Buffer, 2.0ml/vial; 4 CSE; 2 Water for BET, 50ml/vial; |
0.005-5 EU/ml |
|
KC0817S |
0.001-10 EU/ml |
3. Product Feature and Application
BioendoTM KC Endotoxin Test Kit (Kinetic Chromogenic Assay) features strong resistance to interference, and has advantages of kinetic turbidimetric and end-point chromogenic method. It is especially suitable for endotoxin detection of biological samples like vaccine, antibody, protein, nucleic acid, etc.
Note:
Lyophilized Amebocyte Lysate reagent manufactured by Bioendo is made from amebocyte lysate from the horseshoe crab (Tachypleus tridentatus).
Product Condition:
The sensitivity of Lyophilized Amebocyte Lysate and potency of Control Standard Endotoxin are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come with product instruction, Certificate of Analysis.
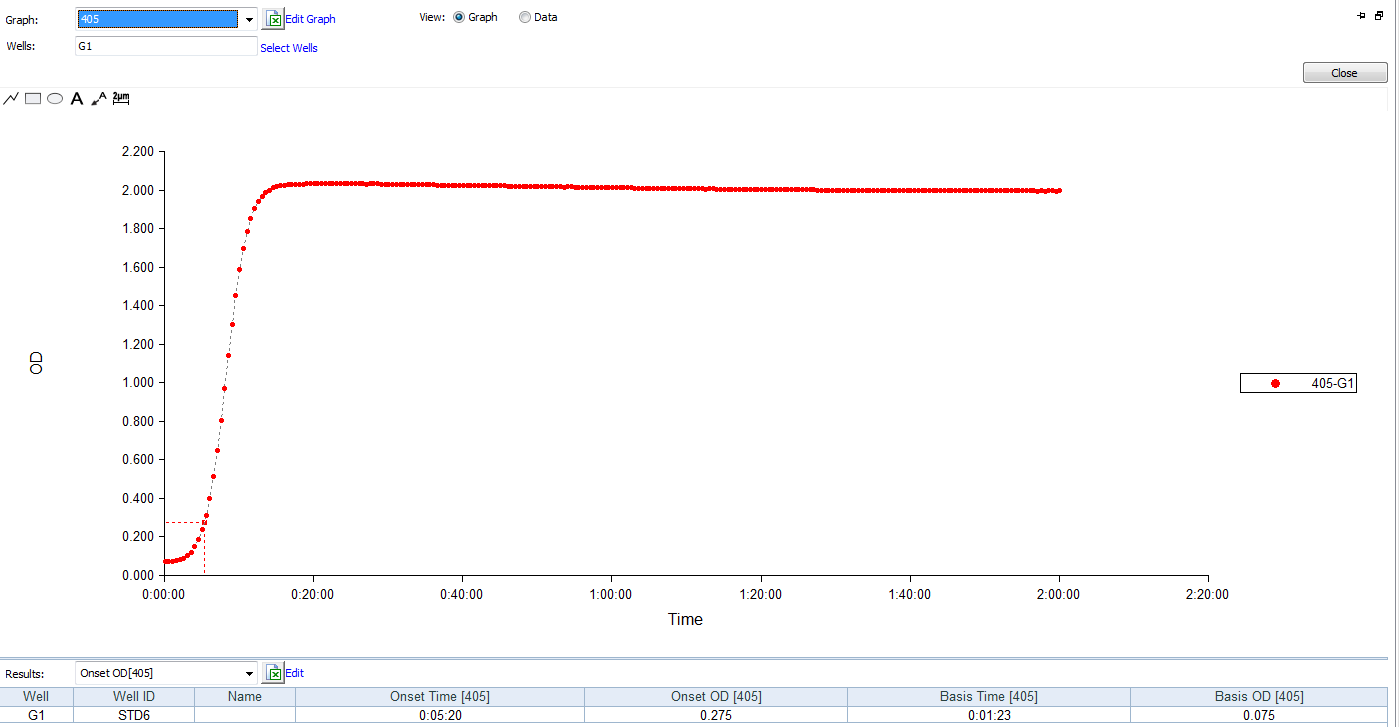
The kinetic chromogenic endotoxin test kit have to choose the microplate reader with 405nm filters.

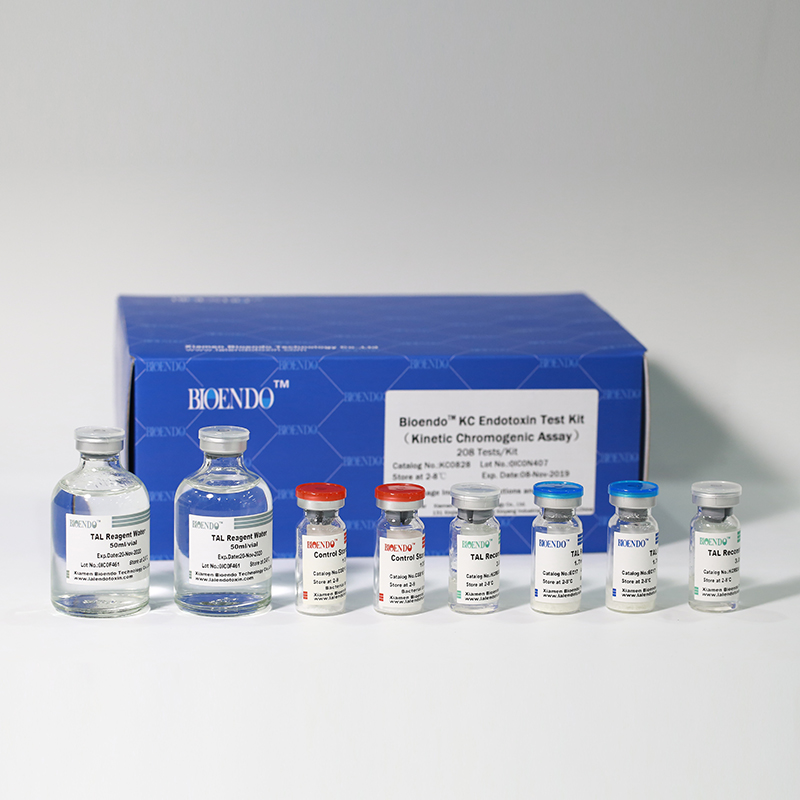
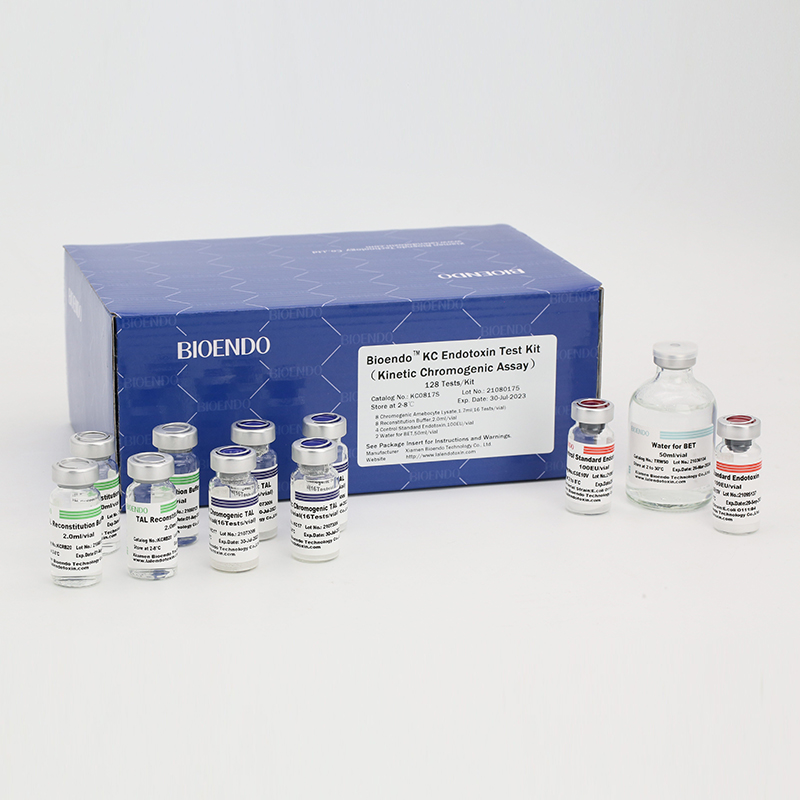
Product detail pictures:



Related Product Guide:
Our personnel are generally in the spirit of "continuous improvement and excellence", and together with the outstanding top quality merchandise, favorable price tag and fantastic after-sales solutions, we try to gain every single customer's rely on for Good quality Micro kinetic chromogenic endotoxin test kit - Bioendo KC Endotoxin Test Kit (Kinetic Chromogenic Assay) – Bioendo , The product will supply to all over the world, such as: United Arab Emirates, Palestine, Madagascar, We now have been making our goods for more than 20 years . Mainly do wholesale , so we've the most competitive price , but highest quality. For the past years , we got very good feedbacks , not only because we offer good solutions , but also because of our good after-sale service . We are here waiting for yourself for your inquiry.
In general, we are satisfied with all aspects, cheap, high-quality, fast delivery and good procuct style, we will have follow-up cooperation!