Factory supplied Bacterial Endotoxins – Control Standard Endotoxin (CSE) – Bioendo
Factory supplied Bacterial Endotoxins – Control Standard Endotoxin (CSE) – Bioendo Detail:
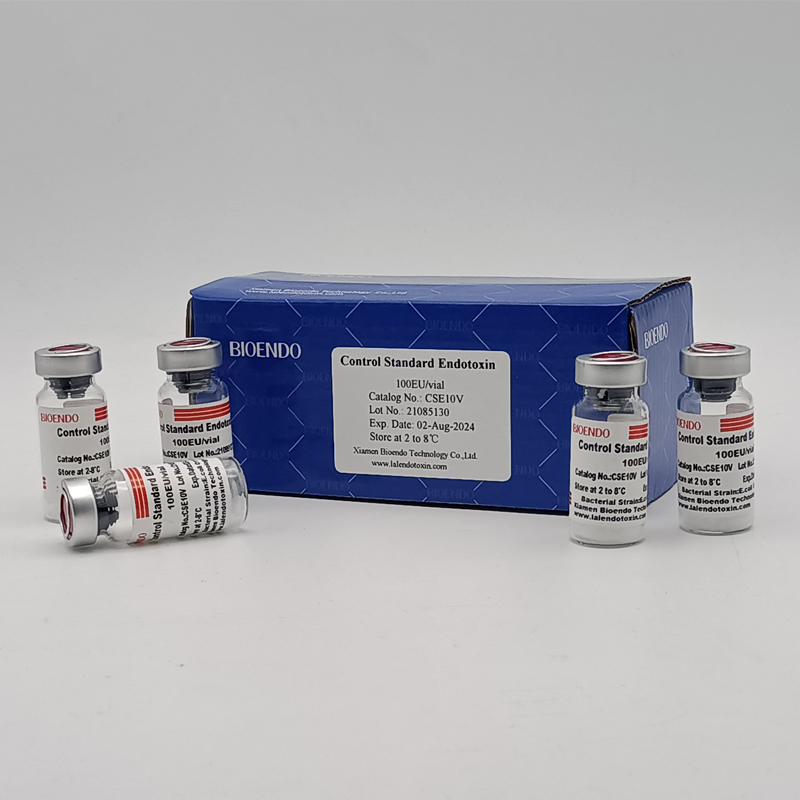
Control Standard Endotoxin (CSE)
1. Product Information
Control Standard Endotoxin (CSE) is extracted from E.coli O111:B4. CSE is an economic alternative to Reference Standard Endotoxin (RSE) in constructing standard curves, validating product and preparing controls in Lyophilized Amebocyte Lysate test. The labeled potency of CSE endotoxinE.coli standard is referenced against RSE. The Control Standard Endotoxin could be used with gel clot assay, kinetic turbidimetric assay or kinetic chromogenic assay as the endotoxin testing standards. The Certificate of Analysis will show the matched Lyophilized Amebocyte Lysate reagent lots.
2. Product Parameter
| Catalog Number | Potency (EU/vial) | Package |
| CSE10V | 100 to 999 EU | seal in glass vial, 10vials/pack |
| CSE100V | 1 to 199 EU | seal in glass vial, 10vials/pack |
| CSE10A | 1 to 99 EU | seal in glass ampoule, 10vials/pack |
3. Product Feature and Application
Bioendo CSE was labeled by the potency and matched to Lyophilized Amebocyte Lysate reagent lots. Users do not need to do the CSE/RSE ratio assay. Low potency control standard endotoxin is available to avoid lots of steps of dilution to provide convenience for end users.
Product Condition:
Control Standard Endotoxin (CSE), extracted from E.coli O111:B4, is an economic alternative to Reference Standard Endotoxin (RSE) in constructing standard curves, validating product and preparing controls in endotoxin test. The potency of CSE is referenced against USP Reference Standard Endotoxin, and labeled in the Certificate of Analysis.
Endotoxin test assay: Lysate reagent and CSE lot number have to be matched.
Pyrogen free tip box
Endotoxin free tubes
Product detail pictures:


Related Product Guide:
Our primary objective is always to offer our clients a serious and responsible small business relationship, offering personalized attention to all of them for Factory supplied Bacterial Endotoxins – Control Standard Endotoxin (CSE) – Bioendo , The product will supply to all over the world, such as: Lahore, Jamaica, Turkmenistan, Most problems between suppliers and clients are due to poor communication. Culturally, suppliers can be reluctant to question things they do not understand. We break down those barriers to ensure you get what you want to the level you expect, when you want it. Faster delivery time and the product you want is our Criterion .
The company can keep up with the changes in this industry market, product updates fast and the price is cheap, this is our second cooperation, it's good.