Chinese Professional LAL testing medical devices - Endotoxin Assay Kit for Human Plasma – Bioendo
Chinese Professional LAL testing medical devices - Endotoxin Assay Kit for Human Plasma – Bioendo Detail:
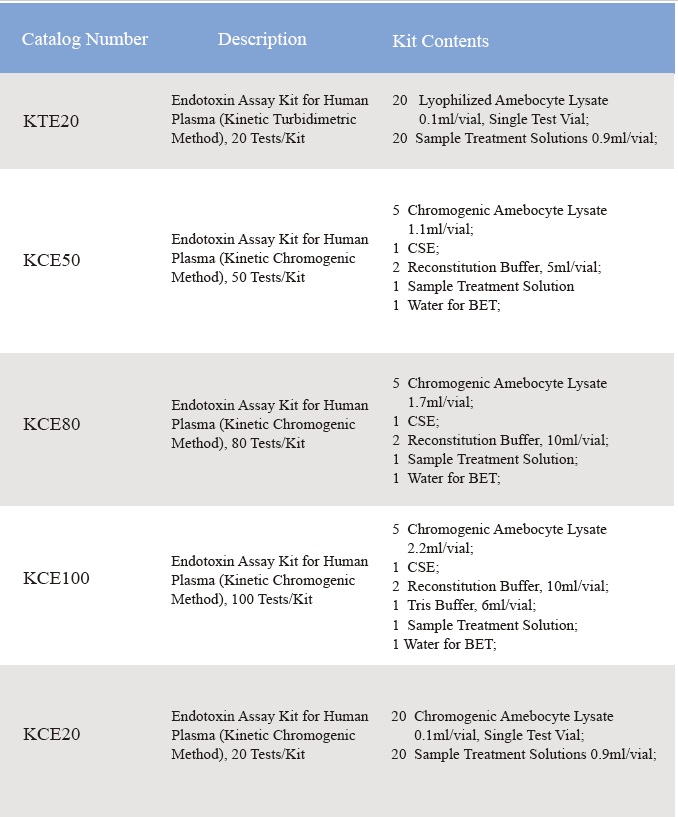
Endotoxin Assay Kit for Human Plasma
1. Product Information
CFDA cleared Clinical diagnostic Endotoxin assay kit quantifies endotoxin level inhuman plasma. Endotoxin is a major component of the cell wall of Gram Negative bacteria and is the most important microbial mediator of sepsis. Elevated levels of endotoxin can often induce fever, changes in white blood cell countsand, in some cases, cardiovascular shock. It is based on the factor Cpathway in limulus Polyphemus (horseshoe crab blood) test. With kinetic microplate reader and endotoxin assay software, Endotoxin assay kit detects endotoxin level in human plasma in less than one hour. The kit comes with the plasma pre-treatment reagent that eliminates the inhibition factors in plasma during the endotoxin assay.
2. Product Parameter
Assay range: 0.01-10 EU/ml
3. Product Feature and Application
Comes with plasma pretreatment solutions, eliminates the inhibition factors in the human plasma.
Note:
Lyophilized Amebocyte Lysate (LAL) reagent manufactured by Bioendo is made from amebocyte lysate derived blood of horseshoe crab.
The sensitivity of Lyophilized Amebocyte Lysate and potency of Control Standard Endotoxin are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come with product instruction, Certificate of Analysis, MSDS.
Product detail pictures:
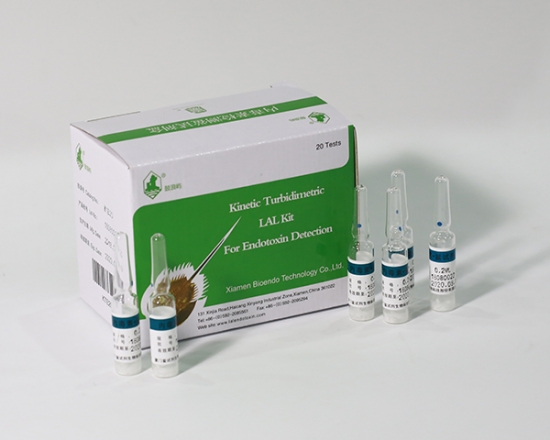

Related Product Guide:
Our eternal pursuits are the attitude of "regard the market, regard the custom, regard the science" as well as the theory of "quality the basic, have confidence in the very first and management the advanced" for Chinese Professional LAL testing medical devices - Endotoxin Assay Kit for Human Plasma – Bioendo , The product will supply to all over the world, such as: Manila, Afghanistan, Romania, Our company covers an area of 20, 000 square meters. We have more than 200 workers, professional technical team, 15 years' experience, exquisite workmanship, stable and reliable quality, competitive price and sufficient production capacity, this is how we make our customers stronger. If you have any inquiry, please do not hesitate to contact us.
Good quality and fast delivery, it's very nice. Some products have a little bit problem, but the supplier replaced timely, overall, we are satisfied.







