Chinese Professional endotoxin removal - EtEraser™ HP Endotoxin Removal Kit – Bioendo
Chinese Professional endotoxin removal - EtEraser™ HP Endotoxin Removal Kit – Bioendo Detail:
EtEraser™ SE Endotoxin Removal Kit
1. Product information
We are the expert of endotoxin testing and endotoxinremoval. We offer the complete solutions covering the whole range of endotoxincontrol, endotoxin monitoring and testing. Our products include laboratoryresearch uses endotoxin removal kits and could be scaled up to industrialproduction scale columns. Lipopolysaccharide (LPS) is a bacterial endotoxin anda major constituent of the cell walls of gram-negative bacteria. Recombinantprotein fromE.coliusually contains high level of endotoxins. Theremoval of these endotoxins is highly necessary for downstream processes.
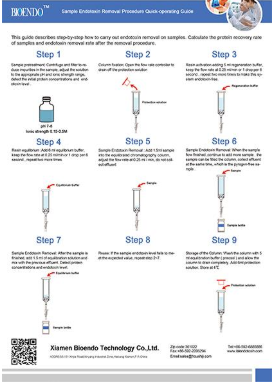
EtEraser HP Endotoxin Removal Kit are designed to remove endotoxincontamination from aqueous solutions. The kits contain endotoxin removal resinbinds to reduce endotoxin levels in protein samples by ≥99% in less than 2hours.
This endotoxin removal kit canbe used in protein, DNA/RNA, polysaccharide andother biological samples.
2.Product features
◆ high stability — does not affectthe activity of most of the biological sample
◆ high protein recovery — >95%protein recovery for protein samples
◆ high removal efficiency — remove>99% endotoxin,endotoxin level in the sample can be less than 0.1 EU/mlafter endotoxin removing process
◆ wide application range — can beused in endotoxin removal for proteins, peptides, antibodies, vaccines,polysaccharide and other biological samples
EtEraserHP High Efficient endotoxin removal kit utilizes immobilized affinity ligandmodified PMB to bind and remove endotoxin from aqueous solution. The modifiedPMB (polymyxin B) ligand is a high specific endotoxin binding ligand. Thecolumn has very high protein recovery rate for more than 95%. Endotoxin levelin the sample can be less than 0.1 EU/ml after endotoxin removing process.
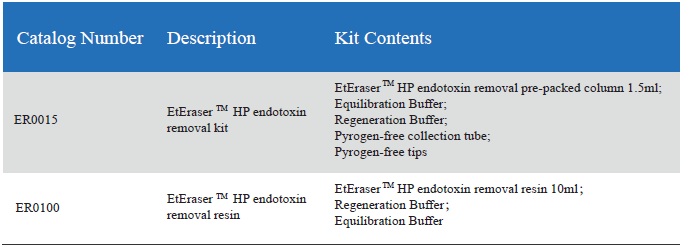
The kitincludes a pre-packed endotoxin removal column 1.5 ml, Equilibration Buffer,Regeneration Buffer and pyrogen-free collection tube and tips. The column has ahigh binding capacity of > 2, 000, 000 EU / ml. This product may be reusedup to five times if properly regenerated. The affinity resin is available inslurry and could be up-scaling in biopharmaceutical process.

Product detail pictures:


Related Product Guide:
Our advantages are lower prices,dynamic sales team,specialized QC,strong factories,high quality products and services for Chinese Professional endotoxin removal - EtEraser™ HP Endotoxin Removal Kit – Bioendo , The product will supply to all over the world, such as: Accra, Niger, Iraq, We seriously promise that we provide all the customers with the best quality products, the most competitive prices and the most prompt delivery. We hope to win a resplendent future for customers and ourselves.
Company director has very rich management experience and strict attitude, sales staff are warm and cheerful, technical staff are professional and responsible,so we have no worry about product,a nice manufacturer.