Bottom price 250ul Pipette tip box - Rapid Gel Clot 10 Samples Kit – Bioendo
Bottom price 250ul Pipette tip box - Rapid Gel Clot 10 Samples Kit – Bioendo Detail:
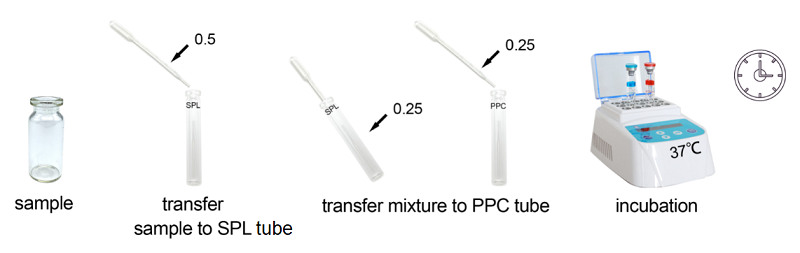
Bioendo Rapid Gel Clot Endotoxin Assay Kit is designed to rapidly quantify endotoxin in water or dialysate. Generally, RG kit’s result could be gained within 30 minutes. Under the guidance of detecting endotoxin in water or dialysate quickly, endotoxin detection with Bioendo Rapid Gel Clot Endotoxin Assay Kit does not need the multi steps’ dilution of Control Standard Endotoxin and test samples. Operation procedures are very convenient, additional experimental equipment are required. It is a convenient and rapid way to detect endotoxin in especial suitable for water or dialysate.
2. Product Parameter
Sensitivity Range: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25EU/ml, 0.5EU/ml
10 sample tests in the kit.
Assay time: less than 30 minutes
3. Product Application
Bioendo Rapid Gel Clot Endotoxin Assay Kit is designed to rapidly quantify endotoxin in water or dialysate as well as do quickly endotoxin detection in life science research.
Note:
Lyophilized Amebocyte Lysate (LAL) reagent manufactured by Bioendo is made from amebocyte lysate derived blood of horseshoe crab.
|
Catalog No. |
Description |
Kit Contents |
Sensitivity EU/ml |
Reaction Time minutes |
|
RG10025003 |
BioendoTM Rapid Gel Clot Endotoxin Assay Kit, 10 Samples Kit |
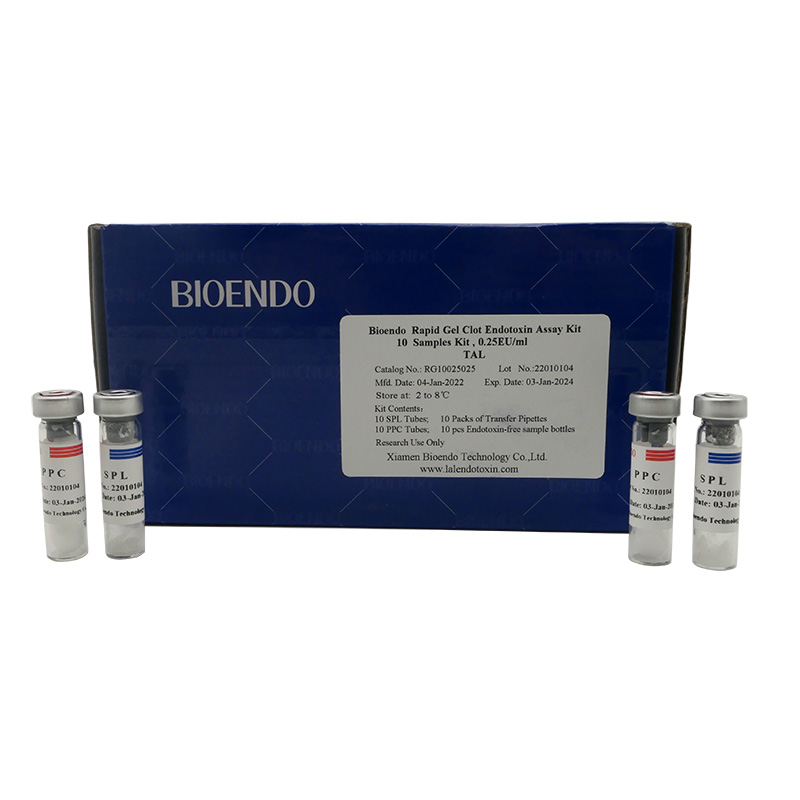
10 SPL Tubes; 10 PPC Tubes; 10 Endotoxin-free Sample Bottles; 10 Packs of (3pcs Transfer Pipettes) |
0.03 |
≤60 |
|
RG10025006 |
0.06 |
≤60 |
||
|
RG100250125 |
0.125 |
≤45 |
||
|
RG10025025 |
0.25 |
≤30 |
||
|
RG10025050 |
0.5 |
≤30 |
Product detail pictures:



Related Product Guide:
We have quite a few great team customers very good at internet marketing, QC, and dealing with kinds of troublesome trouble while in the output approach for Bottom price 250ul Pipette tip box - Rapid Gel Clot 10 Samples Kit – Bioendo , The product will supply to all over the world, such as: Jamaica, Swiss, Rotterdam, Our company always concentrate on the development of the international market. We've got a lot of customers in Russia , European countries, the USA, the Middle East countries and Africa countries. We always follow that quality is foundation while service is guarantee to meet all customers.
High Quality, High Efficiency, Creative and Integrity, worth having long-term cooperation! Looking forward to the future cooperation!








