Best quality LAL Reagent Water - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo
Best quality LAL Reagent Water - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo Detail:
Single-Channel Mechanical Pipettes (Semi-sterile)
1. Product Information
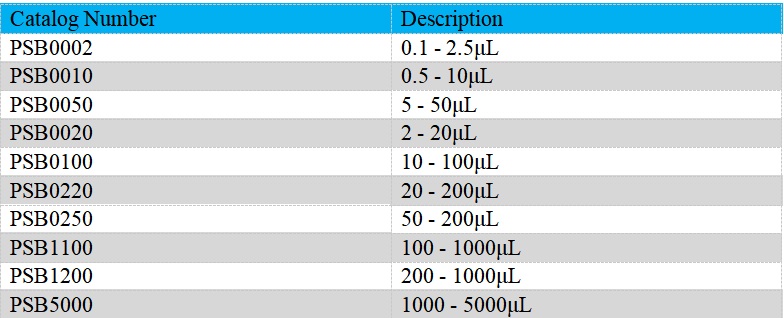
The Single-Channel Mechanical Pipettes (Semi-sterile) is adjustable, which could be used to dispense liquid precisely. Measuring volume of our Single-Channel Mechanical Pipettes (Semi-sterile) ranges from 0.1μL to 5mL. Products are produced based on ISO8655/DIN12650. It could be widely employed on endotoxin detection, etc..
2. Product Features
- Light weight, economic
- The pipettes cover volume range from 0.1μL to 5mL
- Semi-sterile
- Easy calibration and maintenance
Product detail pictures:

Related Product Guide:
"Based on domestic market and expand overseas business" is our development strategy for Best quality LAL Reagent Water - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo , The product will supply to all over the world, such as: Washington, Bangkok, Iceland, As an experienced factory we also accept customized order and make it same as your picture or sample specifying specification and customer design packing. The main goal of the company is to live a satisfactory memory to all the customers,and establish a long term win-win business relationship. For more information,please contact us. And it's our great pleasure if you like to have a personally meeting in our office.
The sales manager is very patient, we communicated about three days before we decided to cooperate, finally, we are very satisfied with this cooperation!








