2022 wholesale price Kinetic Turbidimetric vs Kinetic Chromogenic - Bioendo KC Endotoxin Test Kit (Kinetic Chromogenic Assay) – Bioendo
2022 wholesale price Kinetic Turbidimetric vs Kinetic Chromogenic - Bioendo KC Endotoxin Test Kit (Kinetic Chromogenic Assay) – Bioendo Detail:
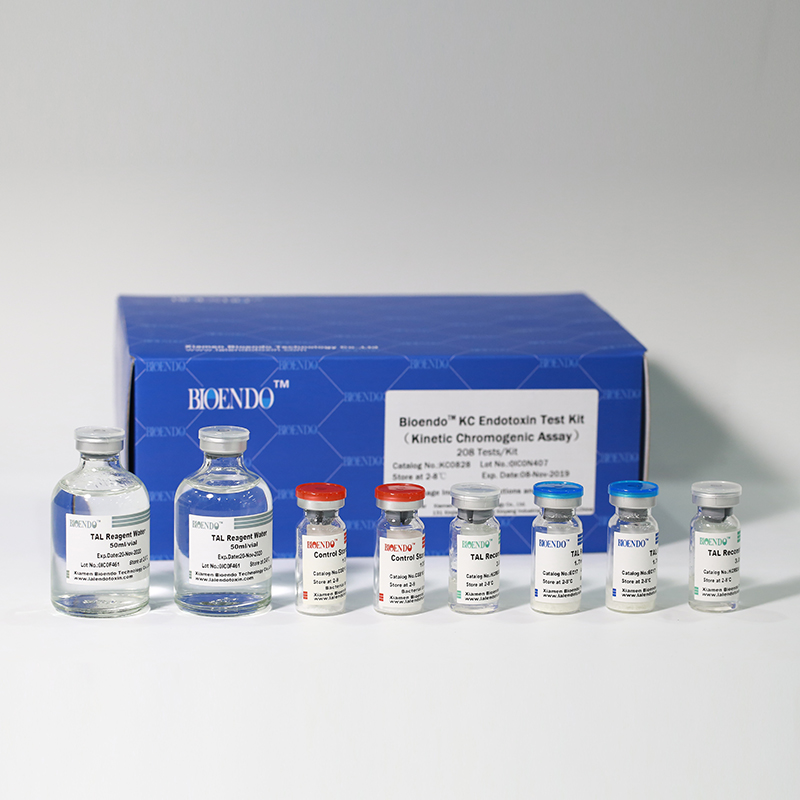
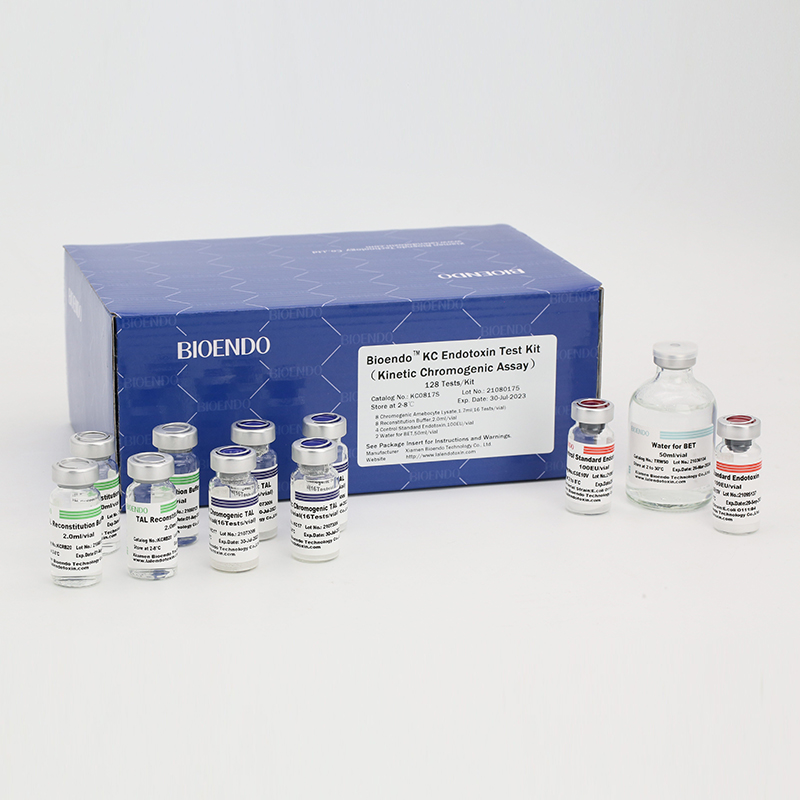
Bioendo KC Endotoxin Test Kit (Kinetic Chromogenic Assay)
1. Product Information
In Bioendo KC Endotoxin Test Kit, Amebocyte Lysate is co-lyophilized with chromogenic substrate. Therefore, bacterial endotoxin could be quantified based on the chromogenic reaction. The assay is strong resistance to interference, and has advantages of kinetic turbidimetric and end-point chromogenic method. Bioendo Endotoxin Test Kit contains Chromogenic Amebocyte Lysate, Reconstitution Buffer, CSE, Water for BET. Endotoxin detection with Kinetic Chromogenic method requires a kinetic incubating microplate reader such as ELx808IULALXH.
2. Product Parameter
Assay Range: 0.005 – 50EU/ml; 0.001 – 10EU/ml
|
Catalog No. |
Description |
Kit Contents |
Sensitivity EU/ml |
|
KC5028 |
Bioendo™ KC Endotoxin Test Kit (Kinetic Chromogenic Assay), 1300 Tests/Kit |
50 Chromogenic Amebocyte Lysate, 2.8ml (26 Tests/Vial); 50 Reconstitution Buffer, 3.0ml/vial; 10CSE; |
0.005-5EU/ml |
|
KC5028S |
0.001-10EU/ml |
||
|
KC0828 |
Bioendo™ KC Endotoxin Test Kit (Kinetic Chromogenic Assay), 208 Tests/Kit |
8 Chromogenic Amebocyte Lysate, 2.8ml (26 Tests/Vial); 8 Reconstitution Buffer, 3.0ml/vial; 4 CSE; 2 Water for BET, 50ml/vial; |
0.005-5EU/ml |
|
KC0828S |
0.001-10EU/ml |
||
|
KC5017 |
Bioendo™ KC Endotoxin Test Kit (Kinetic Chromogenic Assay), 800 Tests/Kit |
50 Chromogenic Amebocyte Lysate, 1.7ml (16 Tests/Vial); 50 Reconstitution Buffer, 2.0ml/vial; 10CSE; |
0.005-5 EU/ml |
|
KC5017S |
0.001-10 EU/m |
||
|
KC0817 |
Bioendo™ KC Endotoxin Test Kit (Kinetic Chromogenic Assay), 128 Tests/kit |
8 Kinetic Chromogenic Amebocyte Lysate, 1.7ml (16 Tests/vial); 8 Reconstitution Buffer, 2.0ml/vial; 4 CSE; 2 Water for BET, 50ml/vial; |
0.005-5 EU/ml |
|
KC0817S |
0.001-10 EU/ml |
3. Product Feature and Application
BioendoTM KC Endotoxin Test Kit (Kinetic Chromogenic Assay) features strong resistance to interference, and has advantages of kinetic turbidimetric and end-point chromogenic method. It is especially suitable for endotoxin detection of biological samples like vaccine, antibody, protein, nucleic acid, etc.
Note:
Lyophilized Amebocyte Lysate reagent manufactured by Bioendo is made from amebocyte lysate from the horseshoe crab (Tachypleus tridentatus).
Product Condition:
The sensitivity of Lyophilized Amebocyte Lysate and potency of Control Standard Endotoxin are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come with product instruction, Certificate of Analysis.
The kinetic chromogenic endotoxin test kit have to choose the microplate reader with 405nm filters.


Product detail pictures:



Related Product Guide:
we can easily offer you high-quality products and solutions, competitive rate and very best shopper support. Our destination is "You come here with difficulty and we give you a smile to take away" for 2022 wholesale price Kinetic Turbidimetric vs Kinetic Chromogenic - Bioendo KC Endotoxin Test Kit (Kinetic Chromogenic Assay) – Bioendo , The product will supply to all over the world, such as: New York, Honduras, Bangladesh, Based on our guiding principle of quality is the key to development, we continually strive to exceed our customers' expectations. As such, we sincerely invite all interested companies to contact us for future cooperation, We welcome old and new customers to hold hands together for exploring and developing; For more information, be sure to feel free to contact us. Thanks. Advanced equipment, strict quality control, customer-orientation service, initiative summary and improvement of defects and extensive industry experience enable us to guarantee more customer satisfaction and reputation which, in return, brings us more orders and benefits. If you are interested in any of our merchandise, make sure you feel free to contact us. Inquiry or visit to our company are warmly welcome. We sincerely hope to start a win-win and friendly partnership with you. You can see more details in our website.
This supplier stick to the principle of "Quality first, Honesty as base", it is absolutely to be trust.








