2022 High quality LAL assay kit - Mini Dry Heat Incubator – Bioendo
2022 High quality LAL assay kit - Mini Dry Heat Incubator – Bioendo Detail:
Dry heat incubator single module
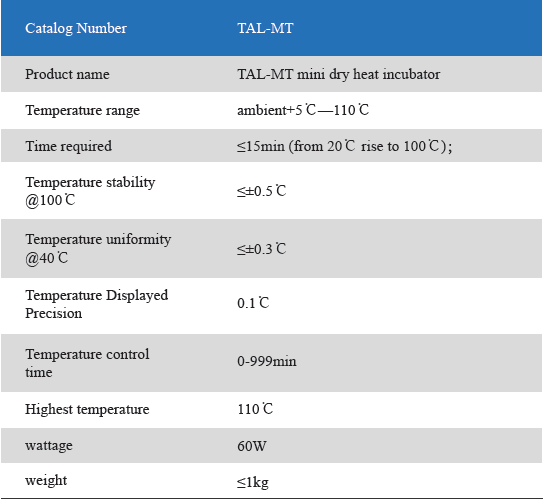
1. Product information
The Mini Dry Heat Incubator is a micro-processor controlled heating block with semi conductor heating technology.It adapts onboard use, smart, light and convenient for movement, suit for any kind of occasions. Especially good for the incubation of the gel clot LAL assay, LAL chromogenic endpoint assay incubation.
2. Product features
1. Unique designed. Smart and light, convenient movement, suit for various occasions.
2. LCD simultaneously display setting and actual time and temperature.Temperature calibration function.
3. Automatic fault detection function with buzzer alarm.
4. 24V DC input power, built-in over-temperature protection device.
5. Various of blocks for optional choice. Convenient for replacement. Easy cleaning and disinfection.
Product detail pictures:



Related Product Guide:
We are ready to share our knowledge of marketing worldwide and recommend you suitable products at most competitive prices. So Profi Tools offer you best value of money and we are ready to develop together with 2022 High quality LAL assay kit - Mini Dry Heat Incubator – Bioendo , The product will supply to all over the world, such as: Guatemala, Qatar, Denver, We are proud to supply our products to every costumer all around the world with our flexible, fast efficient services and strictest quality control standard which has always approved and praised by customers.
In China, we have purchased many times, this time is the most successful and most satisfactory, a sincere and realiable Chinese manufacturer!